Chromosome 1q gain and tenascin-C expression are candidate markers to define different risk groups in pediatric posterior fossa ependymoma
- PMID: 27550150
- PMCID: PMC4994287
- DOI: 10.1186/s40478-016-0349-9
Chromosome 1q gain and tenascin-C expression are candidate markers to define different risk groups in pediatric posterior fossa ependymoma
Abstract
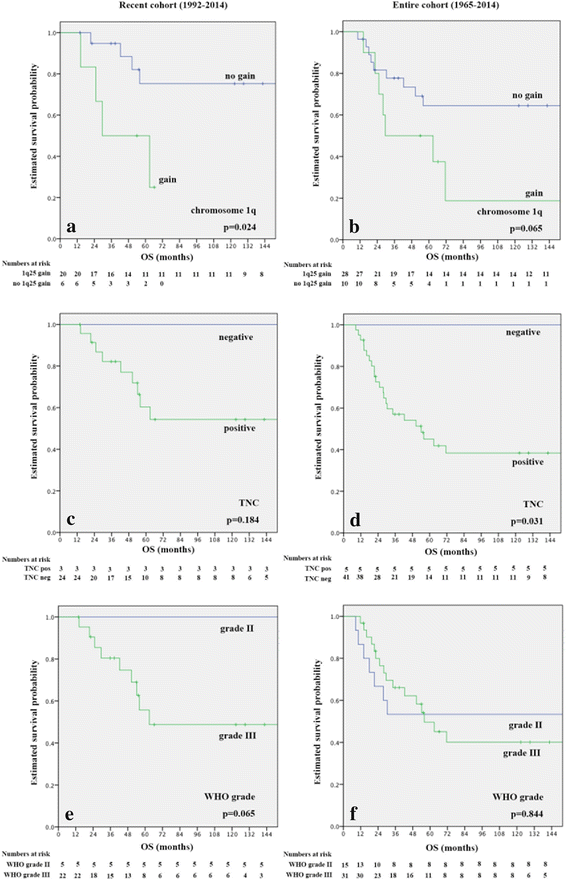
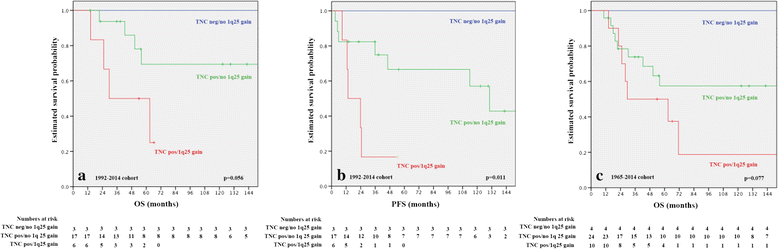
Intracranial classic (WHO grade II) and anaplastic (WHO grade III) ependymomas are among the most common tumors in pediatric patients and have due to frequent recurrences and late relapses a relatively poor outcome. The impact of histopathological grading on patient outcome is controversial and therefore, molecular prognostic and predictive markers are needed to improve patient outcome. To date, the most promising candidate marker is chromosome 1q gain, which has been associated in independent studies with adverse outcome. Furthermore, gene expression and methylation profiles revealed distinct molecular subgroups in the supratentorial and posterior fossa (PF) compartment and Laminin alpha-2 (LAMA2) and Neural Epidermal Growth Factor Like-2 (NELL2) were suggested as surrogate markers for the two PF subgroups PF-EPN-A and PF-EPN-B. PF-EPN-A tumors were also characterized by tenascin-C (TNC) expression and tenascin-C has been suggested as candidate gene on 9q, involved in tumor progression. Therefore, we have analyzed the status of chromosome 1q, TNC, LAMA2, and NELL2 expression in a series of pediatric PF ependymomas in terms of their frequency, associations among themselves, and clinical parameters, as well as their prognostic impact. We confirm the negative prognostic impact of 1q gain and TNC expression and could classify PF ependymomas by these two markers into three molecular subgroups. Tumors with combined 1q gain and TNC expression had the poorest, tumors without 1q gain and TNC expression had a favorable and TNC positive 1q non-gained cases had an intermediate outcome. We found also differences in age and tumor grade in the three subgroups and thus, provide evidence that PF pediatric ependymomas can be divided by chromosome 1q status and TNC expression in three molecular subgroups with distinct clinico-pathological features. These analyses require only few amounts of tumor tissue, are broadly available in the routine clinical neuropathological setting and thus, could be used in further therapy trials to optimize treatment of ependymoma patients.
Keywords: Chromosome 1q; Ependymoma; Pediatric; Prognostic markers; Tenascin-C.
Figures

References
-
- Ostrom QT, de Blank PM, Kruchko C, Petersen CM, Liao P, Finlay JL, Stearns DS, Wolff JE, Wolinsky Y, Letterio JJ, Barnholtz-Sloan JS. Alex’s Lemonade Stand Foundation Infant and Childhood Primary Brain and Central Nervous System Tumors Diagnosed in the United States in 2007-2011. Neuro Oncol. 2015;16(Suppl 10):x1–x36. doi: 10.1093/neuonc/nou327. - DOI - PMC - PubMed
-
- Grundy RG, Wilne SA, Weston CL, Robinson K, Lashford LS, Ironside J, Cox T, Chong WK, Campbell RH, Bailey CC, Gattamaneni R, Picton S, Thorpe N, Mallucci C, English MW, Punt JA, Walker DA, Ellison DW, Machin D. Primary postoperative chemotherapy without radiotherapy for intracranial ependymoma in children: the UKCCSG/SIOP prospective study. Lancet Oncol. 2007;8:696–705. doi: 10.1016/S1470-2045(07)70208-5. - DOI - PubMed
-
- Massimino M, Gandola L, Giangaspero F, Sandri A, Valagussa P, Perilongo G, Garre M-L, Ricardi U, Forni M, Genitori L, Scarzello G, Spreafico F, Barra S, Mascarin M, Pollo B, Gardiman M, Cama A, Navarria P, Brisigotti M, Collini P, Balter R, Fidani P, Stefanelli M, Burnelli R, Potepan P, Podda M, Sotti G, Madon E. Hyperfractionated radiotherapy and chemotherapy for childhood ependymoma: final results of the first prospective AIEOP (Associazione Italiana di Ematologia-Oncologia Pediatrica) study. Int J Radiat Oncol Biol Phys. 2004;58:1336–1345. doi: 10.1016/j.ijrobp.2003.08.030. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

