Ethanolic extract of Schizonepeta tenuifolia attenuates osteoclast formation and activation in vitro and protects against lipopolysaccharide-induced bone loss in vivo
- PMID: 27550314
- PMCID: PMC4994400
- DOI: 10.1186/s12906-016-1300-0
Ethanolic extract of Schizonepeta tenuifolia attenuates osteoclast formation and activation in vitro and protects against lipopolysaccharide-induced bone loss in vivo
Abstract
Background: Excessive osteoclast activity is a major cause of metabolic bone disorders, such as osteopenia, rheumatoid arthritis, and osteoporosis. Thus, discovery of agents targeting osteoclast differentiation and bone resorption is important for development of novel treatments for bone diseases. It has been demonstrated that ethanolic extract of schizonepeta tenuifolia (EEST) has potent anti-oxidant and anti-inflammatory activities. However, the beneficial effects of EEST on bone metabolism have not been studied. Therefore, we intend to investigate the effects of EEST on osteoclast differentiation.
Methods: We examined the effects and mechanisms of action of the EEST on osteoclastogenesis in vitro in bone marrow macrophages (BMMs) stimulated with receptor activator of nuclear factor kappa-B ligand (RANKL) and in vivo using a mouse model of lipopolysaccharide (LPS)-induced bone destruction.
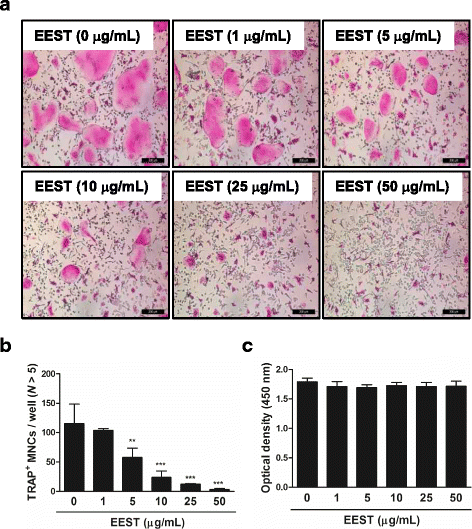
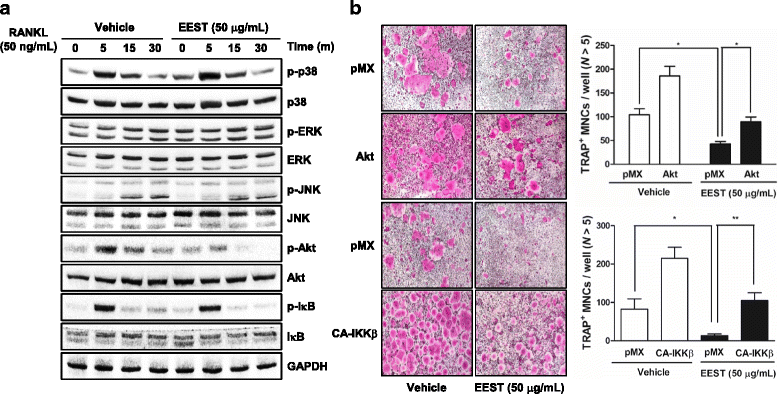
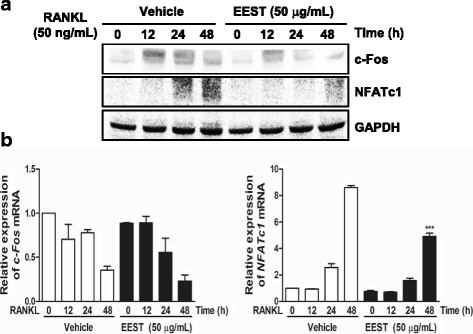
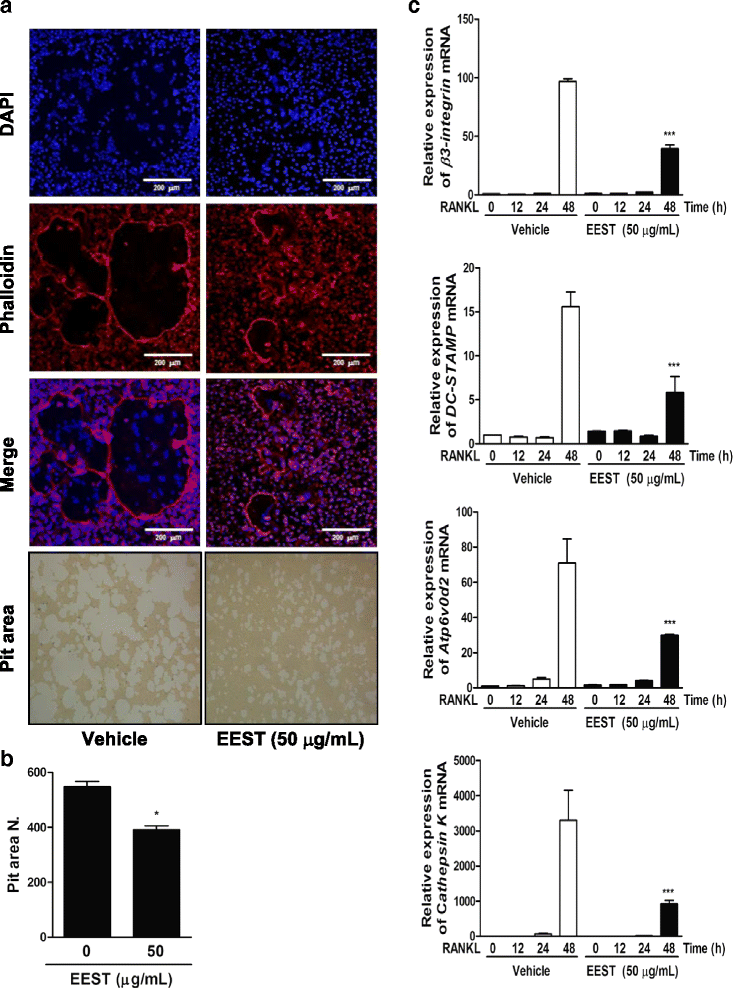
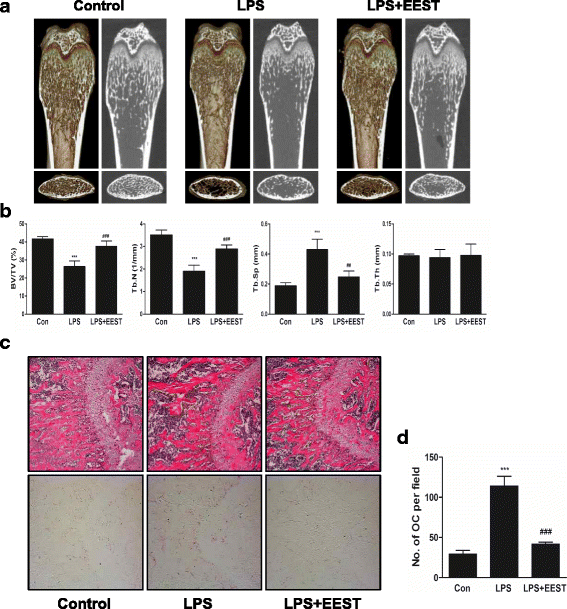
Results: We found that EEST inhibited phosphorylation of Akt and IkB at early stages of RANKL-induced osteoclastogenesis. Furthermore, EEST negatively controlled the transcription and translation levels of nuclear factor of activated T cells c1 (NFATc1) and the translation level of c-Fos at the final stage of osteoclast differentiation. Reflecting these effects, EEST blocked both filamentous actin (F-actin) ring formation and bone resorbing activity of mature osteoclasts in vitro. The inhibitory effects of EEST on osteoclast formation and activity were observed in an LPS-mediated bone erosion mouse model using micro-CT and histological analysis.
Conclusions: EEST is a potential agent that is able to treat osteoclast-related bone diseases, such as osteoporosis.
Keywords: Bone resorption; Osteoclast differentiation; Osteoporosis; Schizonepeta tenuifolia.
Figures
Similar articles
-
Ebselen Is a Potential Anti-Osteoporosis Agent by Suppressing Receptor Activator of Nuclear Factor Kappa-B Ligand-Induced Osteoclast Differentiation In vitro and Lipopolysaccharide-Induced Inflammatory Bone Destruction In vivo.Int J Biol Sci. 2016 Feb 18;12(5):478-88. doi: 10.7150/ijbs.13815. eCollection 2016. Int J Biol Sci. 2016. PMID: 27019631 Free PMC article.
-
Purslane suppresses osteoclast differentiation and bone resorbing activity via inhibition of Akt/GSK3β-c-Fos-NFATc1 signaling in vitro and prevents lipopolysaccharide-induced bone loss in vivo.Biol Pharm Bull. 2015;38(1):66-74. doi: 10.1248/bpb.b14-00567. Biol Pharm Bull. 2015. PMID: 25744460
-
Ethanol extract of Polyscias fruticosa leaves suppresses RANKL-mediated osteoclastogenesis in vitro and LPS-induced bone loss in vivo.Phytomedicine. 2019 Jun;59:152908. doi: 10.1016/j.phymed.2019.152908. Epub 2019 Apr 2. Phytomedicine. 2019. PMID: 30981187
-
Advances in osteoclast differentiation and function.Curr Drug Targets Immune Endocr Metabol Disord. 2005 Sep;5(3):347-55. doi: 10.2174/1568008054863808. Curr Drug Targets Immune Endocr Metabol Disord. 2005. PMID: 16178795 Review.
-
Osteoclast function and bone-resorbing activity: An overview.Biochem Biophys Res Commun. 2016 Jul 29;476(3):115-20. doi: 10.1016/j.bbrc.2016.05.019. Epub 2016 May 5. Biochem Biophys Res Commun. 2016. PMID: 27157135 Review.
Cited by
-
7ND protein exerts inhibitory effects on both osteoclast differentiation in vitro and lipopolysaccharide‑induced bone erosion in vivo.Mol Med Rep. 2020 Jul;22(1):97-104. doi: 10.3892/mmr.2020.11119. Epub 2020 May 5. Mol Med Rep. 2020. PMID: 32377737 Free PMC article.
-
Lipopolysaccharide-Induced Bone Loss in Rodent Models: A Systematic Review and Meta-Analysis.J Bone Miner Res. 2023 Jan;38(1):198-213. doi: 10.1002/jbmr.4740. Epub 2022 Dec 5. J Bone Miner Res. 2023. PMID: 36401814 Free PMC article.
-
Review on Chemical Constituents of Schizonepeta tenuifolia Briq. and Their Pharmacological Effects.Molecules. 2022 Aug 17;27(16):5249. doi: 10.3390/molecules27165249. Molecules. 2022. PMID: 36014489 Free PMC article. Review.
-
CGK733 alleviates ovariectomy-induced bone loss through blocking RANKL-mediated Ca2+ oscillations and NF-κB/MAPK signaling pathways.iScience. 2023 Aug 29;26(10):107760. doi: 10.1016/j.isci.2023.107760. eCollection 2023 Oct 20. iScience. 2023. PMID: 37720109 Free PMC article.
-
Poligoni Multiflori Radix enhances osteoblast formation and reduces osteoclast differentiation.Int J Mol Med. 2018 Jul;42(1):331-345. doi: 10.3892/ijmm.2018.3603. Epub 2018 Mar 30. Int J Mol Med. 2018. PMID: 29620250 Free PMC article.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

