Systematic Review and Meta-analysis of the Pharmacokinetics of Benznidazole in the Treatment of Chagas Disease
- PMID: 27550362
- PMCID: PMC5118981
- DOI: 10.1128/AAC.01567-16
Systematic Review and Meta-analysis of the Pharmacokinetics of Benznidazole in the Treatment of Chagas Disease
Abstract
Chagas disease is a neglected parasitic illness affecting approximately 8 million people, predominantly in Latin America. Benznidazole is the drug of choice for treatment, although its availability has been limited. A paucity of knowledge of the pharmacokinetic properties of this drug has contributed to its limited availability in several jurisdictions. The objective of this study was to conduct a systematic literature review and a Bayesian meta-analysis of pharmacokinetic studies to improve estimates of the basic pharmacokinetic properties of benznidazole. A systematic search of the Embase, Medline, LILACS, and SciELO (Scientific Electronic Library Online) databases was conducted. Eligible studies reported patient-level data from single-100-mg-dose pharmacokinetic evaluations of benznidazole in adults or otherwise provided data relevant to the estimation of pharmacokinetic parameters which could be derived from such studies. A Bayesian hierarchical model was used for analysis. Secondary data (i.e., data from studies that did not include patient-level, single-100-mg-dose data) were used for the generation of empirical priors for the Bayesian analysis. The systematic search identified nine studies for inclusion. Nine pharmacokinetic parameters were estimated, including the area under the concentration-time curve (AUC), the maximum concentration of drug in plasma (Cmax), the time to Cmax, the elimination rate constant (kel), the absorption rate constant (Ka), the absorption and elimination half-lives, the apparent oral clearance, and the apparent oral volume of distribution. The results showed consistency across studies. AUC and Cmax were 51.31 mg · h/liter (95% credible interval [CrI], 45.01, 60.28 mg · h/liter) and 2.19 mg/liter (95% CrI, 2.06, 2.33 mg/liter), respectively. Ka and kel were 1.16 h-1 (95% CrI, 0.59, 1.76 h-1) and 0.052 h-1 (95% CrI, 0.045, 0.059 h-1), respectively, with the corresponding absorption and elimination half-lives being 0.60 h (95% CrI, 0.38, 1.11 h) and 13.27 h (95% CrI, 11.79, 15.42 h), respectively. The oral clearance and volume of distribution were 2.04 liters/h (95% CrI, 1.77, 2.32 liters/h) and 39.19 liters (95% CrI, 36.58, 42.17 liters), respectively. A Bayesian meta-analysis was used to improve the estimates of the standard pharmacokinetic parameters of benznidazole. These data can inform clinicians and policy makers as access to this drug increases.
Copyright © 2016, American Society for Microbiology. All Rights Reserved.
Figures

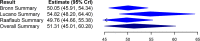
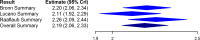

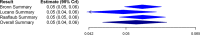



References
-
- World Health Organization. 2016. Chagas disease (American trypanosomiasis). World Health Organization, Geneva, Switzerland: http://www.who.int/mediacentre/factsheets/fs340/en/ Accessed 3 May 2016.
-
- Bern C, Montgomery SP, Herwaldt BL, Rassi A Jr, Marin-Neto JA, Dantas RO, Maguire JH, Acquatella H, Morillo C, Kirchhoff LV, Gilman RH, Reyes PA, Salvatella R, Moore AC. 2007. Evaluation and treatment of Chagas disease in the United States: a systematic review. JAMA 298:2171–2181. doi:10.1001/jama.298.18.2171. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

