WWOX and p53 Dysregulation Synergize to Drive the Development of Osteosarcoma
- PMID: 27550453
- PMCID: PMC5146760
- DOI: 10.1158/0008-5472.CAN-16-0621
WWOX and p53 Dysregulation Synergize to Drive the Development of Osteosarcoma
Abstract
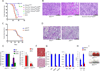
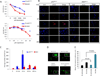
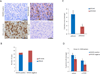
Osteosarcoma is a highly metastatic form of bone cancer in adolescents and young adults that is resistant to existing treatments. Development of an effective therapy has been hindered by very limited understanding of the mechanisms of osteosarcomagenesis. Here, we used genetically engineered mice to investigate the effects of deleting the tumor suppressor Wwox selectively in either osteoblast progenitors or mature osteoblasts. Mice with conditional deletion of Wwox in preosteoblasts (WwoxΔosx1) displayed a severe inhibition of osteogenesis accompanied by p53 upregulation, effects that were not observed in mice lacking Wwox in mature osteoblasts. Deletion of p53 in WwoxΔosx1 mice rescued the osteogenic defect. In addition, the Wwox;p53Δosx1 double knockout mice developed poorly differentiated osteosarcomas that resemble human osteosarcoma in histology, location, metastatic behavior, and gene expression. Strikingly, the development of osteosarcomas in these mice was greatly accelerated compared with mice lacking p53 only. In contrast, combined WWOX and p53 inactivation in mature osteoblasts did not accelerate osteosarcomagenesis compared with p53 inactivation alone. These findings provide evidence that a WWOX-p53 network regulates normal bone formation and that disruption of this network in osteoprogenitors results in accelerated osteosarcoma. The Wwox;p53Δosx1 double knockout establishes a new osteosarcoma model with significant advancement over existing models. Cancer Res; 76(20); 6107-17. ©2016 AACR.
©2016 American Association for Cancer Research.
Conflict of interest statement
of Potential Conflicts of Interest. Non
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

