Dermatological adverse events with taxane chemotherapy
- PMID: 27550571
- PMCID: PMC5526115
- DOI: 10.1684/ejd.2016.2833
Dermatological adverse events with taxane chemotherapy
Abstract
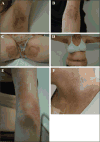
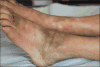
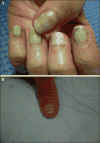
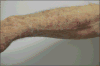
Taxanes (docetaxel and paclitaxel) are among the most commonly prescribed anticancer drugs approved for the treatment of metastatic or locally advanced breast, non-small cell lung, prostate, gastric, head and neck, and ovarian cancers, as well as in the adjuvant setting for operable node-positive breast cancers. Although the true incidence of dermatological adverse events (AEs) in patients receiving taxanes is not known, and has never been prospectively analysed, they clearly represent one of the major AEs associated with these agents. With an increase in the occurrence of cutaneous AEs during treatment with novel targeted and immunological therapies when used in combination with taxanes, a thorough understanding of reactions attributable to this class is imperative. Moreover, identification and management of dermatological AEs is critical for maintaining the quality of life in cancer patients and for minimizing dose modifications of their antineoplastic regimen. This analysis represents a systematic review of the dermatological conditions reported with the use of these drugs, complemented by experience at comprehensive cancer centres. The conditions reported herein include skin, hair, and nail toxicities. Lastly, we describe the dermatological data available for the new, recently FDA-and EMA- approved, solvent-free nab-paclitaxel.
Keywords: docetaxel; hair; nab-paclitaxel; nail; paclitaxel; skin; taxanes; toxicity.
Conflict of interest statement
Conflict of interest: NRL, HR, FD, VRB, AE, LG, MM, MD and EV have no conflicts of interest to declare.
Figures













References
-
- Rowinsky EK, Donehower RC. Paclitaxel (taxol) N Engl J Med. 1995;332:1004–14. - PubMed
-
- Cortes JE, Pazdur R. Docetaxel. J Clin Oncol. 1995;13:2643–55. - PubMed
-
- Gelmon K. The taxoids: paclitaxel and docetaxel. Lancet. 1994;344:1267–72. - PubMed
-
- Markman M. Managing taxane toxicities. Support Care Cancer. 2003;11:144–7. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

