Complex, multi-scale small intestinal topography replicated in cellular growth substrates fabricated via chemical vapor deposition of Parylene C
- PMID: 27550930
- PMCID: PMC8061873
- DOI: 10.1088/1758-5090/8/3/035011
Complex, multi-scale small intestinal topography replicated in cellular growth substrates fabricated via chemical vapor deposition of Parylene C
Abstract
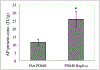
Native small intestine possesses distinct multi-scale structures (e.g., crypts, villi) not included in traditional 2D intestinal culture models for drug delivery and regenerative medicine. The known impact of structure on cell function motivates exploration of the influence of intestinal topography on the phenotype of cultured epithelial cells, but the irregular, macro- to submicron-scale features of native intestine are challenging to precisely replicate in cellular growth substrates. Herein, we utilized chemical vapor deposition of Parylene C on decellularized porcine small intestine to create polymeric intestinal replicas containing biomimetic irregular, multi-scale structures. These replicas were used as molds for polydimethylsiloxane (PDMS) growth substrates with macro to submicron intestinal topographical features. Resultant PDMS replicas exhibit multiscale resolution including macro- to micro-scale folds, crypt and villus structures, and submicron-scale features of the underlying basement membrane. After 10 d of human epithelial colorectal cell culture on PDMS substrates, the inclusion of biomimetic topographical features enhanced alkaline phosphatase expression 2.3-fold compared to flat controls, suggesting biomimetic topography is important in induced epithelial differentiation. This work presents a facile, inexpensive method for precisely replicating complex hierarchal features of native tissue, towards a new model for regenerative medicine and drug delivery for intestinal disorders and diseases.
Figures
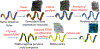




References
-
- Park K, Bass D. Inflammatory bowel diseaseattributable costs and costeffective strategies in the United States: A review. Inflammatory Bowel Diseases. 2011;17(7):1603–9. - PubMed
-
- Buchman AL. Etiology and initial management of short bowel syndrome. Gastroenterology. 2006;130(2 Suppl 1):S5–S15. - PubMed
-
- Faubion WA Jr, Loftus EV Jr, Harmsen WS, Zinsmeister AR, Sandborn WJ. The natural history of corticosteroid therapy for inflammatory bowel disease: a population-based study. Gastroenterology. 2001;121(2):255–60. - PubMed
-
- Khan KJ, Dubinsky MC, Ford AC, Ullman TA, Talley NJ, Moayyedi P. Efficacy of immunosuppressive therapy for inflammatory bowel disease: a systematic review and meta-analysis. The American journal of gastroenterology. 2011;106(4):630–42. - PubMed
-
- Yee S In vitro permeability across Caco-2 cells (colonic) can predict in vivo (small intestinal) absorption in man—fact or myth. Pharmaceutical research. 1997;14(6):763–6. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
