Autophagy and ubiquitin-proteasome system contribute to sperm mitophagy after mammalian fertilization
- PMID: 27551072
- PMCID: PMC5018771
- DOI: 10.1073/pnas.1605844113
Autophagy and ubiquitin-proteasome system contribute to sperm mitophagy after mammalian fertilization
Abstract
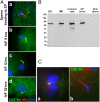
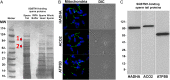
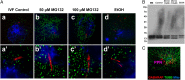
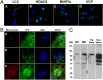
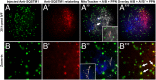
Maternal inheritance of mitochondria and mtDNA is a universal principle in human and animal development, guided by selective ubiquitin-dependent degradation of the sperm-borne mitochondria after fertilization. However, it is not clear how the 26S proteasome, the ubiquitin-dependent protease that is only capable of degrading one protein molecule at a time, can dispose of a whole sperm mitochondrial sheath. We hypothesized that the canonical ubiquitin-like autophagy receptors [sequestosome 1 (SQSTM1), microtubule-associated protein 1 light chain 3 (LC3), gamma-aminobutyric acid receptor-associated protein (GABARAP)] and the nontraditional mitophagy pathways involving ubiquitin-proteasome system and the ubiquitin-binding protein dislocase, valosin-containing protein (VCP), may act in concert during mammalian sperm mitophagy. We found that the SQSTM1, but not GABARAP or LC3, associated with sperm mitochondria after fertilization in pig and rhesus monkey zygotes. Three sperm mitochondrial proteins copurified with the recombinant, ubiquitin-associated domain of SQSTM1. The accumulation of GABARAP-containing protein aggregates was observed in the vicinity of sperm mitochondrial sheaths in the zygotes and increased in the embryos treated with proteasomal inhibitor MG132, in which intact sperm mitochondrial sheaths were observed. Pharmacological inhibition of VCP significantly delayed the process of sperm mitophagy and completely prevented it when combined with microinjection of autophagy-targeting antibodies specific to SQSTM1 and/or GABARAP. Sperm mitophagy in higher mammals thus relies on a combined action of SQSTM1-dependent autophagy and VCP-mediated dislocation and presentation of ubiquitinated sperm mitochondrial proteins to the 26S proteasome, explaining how the whole sperm mitochondria are degraded inside the fertilized mammalian oocytes by a protein recycling system involved in degradation of single protein molecules.
Keywords: autophagy; mitochondria; mitophagy; mtDNA; ubiquitin.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Basse CW. Mitochondrial inheritance in fungi. Curr Opin Microbiol. 2010;13(6):712–719. - PubMed
-
- Sutovsky P, et al. Ubiquitinated sperm mitochondria, selective proteolysis, and the regulation of mitochondrial inheritance in mammalian embryos. Biol Reprod. 2000;63(2):582–590. - PubMed
-
- Sutovsky P, McCauley TC, Sutovsky M, Day BN. Early degradation of paternal mitochondria in domestic pig (Sus scrofa) is prevented by selective proteasomal inhibitors lactacystin and MG132. Biol Reprod. 2003;68(5):1793–1800. - PubMed
-
- Seglen PO, Gordon PB, Holen I. Non-selective autophagy. Semin Cell Biol. 1990;1(6):441–448. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

