Multifaceted Role of Sialylation in Prion Diseases
- PMID: 27551257
- PMCID: PMC4976111
- DOI: 10.3389/fnins.2016.00358
Multifaceted Role of Sialylation in Prion Diseases
Abstract
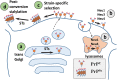
Mammalian prion or PrP(Sc) is a proteinaceous infectious agent that consists of a misfolded, self-replicating state of a sialoglycoprotein called the prion protein, or PrP(C). Sialylation of the prion protein N-linked glycans was discovered more than 30 years ago, yet the role of sialylation in prion pathogenesis remains poorly understood. Recent years have witnessed extraordinary growth in interest in sialylation and established a critical role for sialic acids in host invasion and host-pathogen interactions. This review article summarizes current knowledge on the role of sialylation of the prion protein in prion diseases. First, we discuss the correlation between sialylation of PrP(Sc) glycans and prion infectivity and describe the factors that control sialylation of PrP(Sc). Second, we explain how glycan sialylation contributes to the prion replication barrier, defines strain-specific glycoform ratios, and imposes constraints for PrP(Sc) structure. Third, several topics, including a possible role for sialylation in animal-to-human prion transmission, prion lymphotropism, toxicity, strain interference, and normal function of PrP(C), are critically reviewed. Finally, a metabolic hypothesis on the role of sialylation in the etiology of sporadic prion diseases is proposed.
Keywords: amyloid; neuraminidase; prion disease; prions; sialic acid; sialylation; sialyltransferase; species barrier.
Figures




References
-
- Aminoff D., Bruegge W. F., Bell W. C., Sarpolis K., Williams R. (1977). Role of sialic acid in survival of erythrocytes in the circulation: interaction of neuraminidase-treated and untreated erythrocytes with spleen and liver at the cellular level. Proc. Acad. Natl. Sci. U.S.A. 74, 1521–1524. 10.1073/pnas.74.4.1521 - DOI - PMC - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

