Ammonia as a Potential Neurotoxic Factor in Alzheimer's Disease
- PMID: 27551259
- PMCID: PMC4976099
- DOI: 10.3389/fnmol.2016.00057
Ammonia as a Potential Neurotoxic Factor in Alzheimer's Disease
Abstract
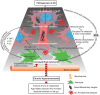
Ammonia is known to be a potent neurotoxin that causes severe negative effects on the central nervous system. Excessive ammonia levels have been detected in the brain of patients with neurological disorders such as Alzheimer disease (AD). Therefore, ammonia could be a factor contributing to the progression of AD. In this review, we provide an introduction to the toxicity of ammonia and putative ammonia transport proteins. We also hypothesize how ammonia may be linked to AD. Additionally, we discuss the evidence that support the hypothesis that ammonia is a key factor contributing to AD progression. Lastly, we summarize the old and new experimental evidence that focuses on energy metabolism, mitochondrial function, inflammatory responses, excitatory glutamatergic, and GABAergic neurotransmission, and memory in support of our ammonia-related hypotheses of AD.
Keywords: Alzheimer disease; GABAergic; ammonia; ammonia transporters; energy metabolism; glutamatergic; mitochondrial dysfunction; toxicity.
Figures
References
-
- Adlimoghaddam A., O'Donnell M. J., Kormish J., Banh S., Treberg J. R., Merz D., et al. . (2016). Ammonia excretion in Caenorhabditis elegans: physiological and molecular characterization of the rhr-2 knock-out mutant. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 195, 46–54. 10.1016/j.cbpa.2016.02.003 - DOI - PubMed
-
- Akude E., Zherebitskaya E., Chowdhury S. K., Smith D. R., Dobrowsky R. T., Fernyhough P. (2011). Diminished superoxide generation is associated with respiratory chain dysfunction and changes in the mitochondrial proteome of sensory neurons from diabetic rats. Diabetes 60, 288–297. 10.2337/db10-0818 - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources