Bacterial Communities: Interactions to Scale
- PMID: 27551280
- PMCID: PMC4976088
- DOI: 10.3389/fmicb.2016.01234
Bacterial Communities: Interactions to Scale
Abstract
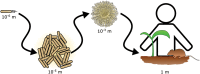
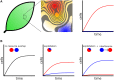
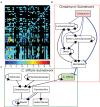
In the environment, bacteria live in complex multispecies communities. These communities span in scale from small, multicellular aggregates to billions or trillions of cells within the gastrointestinal tract of animals. The dynamics of bacterial communities are determined by pairwise interactions that occur between different species in the community. Though interactions occur between a few cells at a time, the outcomes of these interchanges have ramifications that ripple through many orders of magnitude, and ultimately affect the macroscopic world including the health of host organisms. In this review we cover how bacterial competition influences the structures of bacterial communities. We also emphasize methods and insights garnered from culture-dependent pairwise interaction studies, metagenomic analyses, and modeling experiments. Finally, we argue that the integration of multiple approaches will be instrumental to future understanding of the underlying dynamics of bacterial communities.
Keywords: bacterial communities; biodiversity; competition; ecology; interactions; microbiota; scaling; syntrophy.
Figures


References
-
- Alldredge A. L., Silver M. W. (1988). Characteristics, dynamics and significance of marine snow. Prog. Oceanogr. 20 41–82. 10.1016/0079-6611(88)90053-5 - DOI
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

