Temozolomide treatment of pituitary carcinomas and atypical adenomas: systematic review of case reports
- PMID: 27551432
- PMCID: PMC4986928
- DOI: 10.1093/nop/npv059
Temozolomide treatment of pituitary carcinomas and atypical adenomas: systematic review of case reports
Abstract
Background: Pituitary carcinomas (PC) and atypical pituitary adenomas (APA) are rare variants of pituitary tumors for which no evidence-based treatment currently exists. We sought to determine whether temozolomide represents an effective chemotherapeutic option for patients with PC and APA.
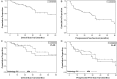
Methods: A systematic review was performed using all published cases of PC and APA treated with temozolomide, and for which information on treatment regimen, clinical response, and survival could be identified. The primary goal of this analysis was to describe overall survival and progression-free survival among PC and APA patients after temozolomide treatment. Secondary goals included assessment of response rate and biomarkers of response.
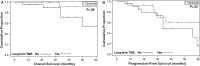
Results: We identified 57 cases and obtained follow-up data on 54 patients (31 APA and 23 PC) for analysis. Estimates of 5-year progression-free survival and overall survival were 21.9% and 57.4% for patients with APA and 36.1% and 56.2% for patients with PC. Among those who responded to temozolomide, overall survival was marginally statistically significantly greater for patients on long-term temozolomide therapy compared with those who were not (5-year overall survival 91.7% vs 54.1%, P = .08); Progression-free survival results were similar but not statistically significant. The objective response rate was 48.4% for patients with APA and 65.2% for patients with PC. Stable disease occurred in 29% of APA and 17.4% of PC patients. Neither histology nor expression of Ki-67 correlated with response; however, negative O6-methylguanine-DNA methyltransferase staining was strongly related to response to temozolomide in patients with APA (P < .001).
Conclusions: Temozolomide is an effective treatment of both PC and APA, and long-term treatment can be considered for particularly aggressive cases.
Keywords: MGMT; atypical pituitary adenoma; pituitary carcinoma; pituitary tumors; temozolomide.
Figures
Similar articles
-
Treatment of pituitary neoplasms with temozolomide: a review.Cancer. 2011 Feb 1;117(3):454-62. doi: 10.1002/cncr.25413. Epub 2010 Sep 15. Cancer. 2011. PMID: 20845485 Review.
-
Temozolomide treatment in aggressive pituitary tumors and pituitary carcinomas: a French multicenter experience.J Clin Endocrinol Metab. 2010 Oct;95(10):4592-9. doi: 10.1210/jc.2010-0644. Epub 2010 Jul 21. J Clin Endocrinol Metab. 2010. PMID: 20660056
-
Lower all-cause mortality rates in patients harboring pituitary carcinoma following the introduction of temozolomide.Endocrine. 2019 Aug;65(2):393-398. doi: 10.1007/s12020-019-01996-9. Epub 2019 Jul 2. Endocrine. 2019. PMID: 31267325
-
DNA mismatch repair protein (MSH6) correlated with the responses of atypical pituitary adenomas and pituitary carcinomas to temozolomide: the national cooperative study by the Japan Society for Hypothalamic and Pituitary Tumors.J Clin Endocrinol Metab. 2013 Mar;98(3):1130-6. doi: 10.1210/jc.2012-2924. Epub 2013 Jan 30. J Clin Endocrinol Metab. 2013. PMID: 23365123
-
The role of temozolomide in the treatment of aggressive pituitary tumors.J Clin Neurosci. 2015 Jun;22(6):923-9. doi: 10.1016/j.jocn.2014.12.007. Epub 2015 Mar 12. J Clin Neurosci. 2015. PMID: 25772801 Review.
Cited by
-
Temozolomide Nonresponsiveness in Aggressive Prolactinomas and Carcinomas: Management and Outcomes.J Endocr Soc. 2021 Dec 22;6(2):bvab190. doi: 10.1210/jendso/bvab190. eCollection 2022 Feb 1. J Endocr Soc. 2021. PMID: 35059545 Free PMC article.
-
Treatment of Aggressive Pituitary Adenomas: A Case-Based Narrative Review.Front Endocrinol (Lausanne). 2021 Nov 15;12:725014. doi: 10.3389/fendo.2021.725014. eCollection 2021. Front Endocrinol (Lausanne). 2021. PMID: 34867776 Free PMC article. Review.
-
Temozolomide and Pituitary Tumors: Current Understanding, Unresolved Issues, and Future Directions.Front Endocrinol (Lausanne). 2018 Jun 15;9:318. doi: 10.3389/fendo.2018.00318. eCollection 2018. Front Endocrinol (Lausanne). 2018. PMID: 29963012 Free PMC article. Review.
-
Combination of PARP inhibitor and temozolomide to suppress chordoma progression.J Mol Med (Berl). 2019 Aug;97(8):1183-1193. doi: 10.1007/s00109-019-01802-z. Epub 2019 Jun 14. J Mol Med (Berl). 2019. PMID: 31201471 Free PMC article.
-
Functioning Pituitary Adenomas - Current Treatment Options and Emerging Medical Therapies.Eur Endocrinol. 2019 Apr;15(1):30-40. doi: 10.17925/EE.2019.15.1.30. Epub 2019 Apr 12. Eur Endocrinol. 2019. PMID: 31244908 Free PMC article. Review.
References
-
- Ezzat S, Asa SL, Couldwell WT et al. . The prevalence of pituitary adenomas. Cancer. 2004;101(3):613–619. - PubMed
-
- DeLellis RA LR, Heitz PU, Eng C. World Health Organization classification of tumours: tumours of endocrine organs. IARC, Lyons. 2004;36–39.
-
- Saeger WLD, Buchfelder M, Fahlbusch R, Quabbe HJ, Petersenn S. Pathohistological classification of pituitary tumors: 10 years of experience with the German Pituitary Tumor Registry. Eur J Endocrinol. 2007;156(2):203–216. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

