Modulation of dendritic cell function by the radiation-mediated secretory protein γ-synuclein
- PMID: 27551446
- PMCID: PMC4979407
- DOI: 10.1038/cddiscovery.2015.11
Modulation of dendritic cell function by the radiation-mediated secretory protein γ-synuclein
Abstract
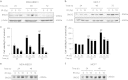
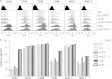
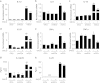
Recently, γ-synuclein (SNCG), which is also known as breast cancer-specific gene-1, has been demonstrated to be an adverse and aggressive marker in breast cancer. In our previous study, SNCG was significantly upregulated in irradiated human breast cancer cells. The aim of this study was to investigate whether radiation-induced, tumor-derived SNCG can influence dendritic cell (DC) function in immune systems. The phenotypical and functional changes of DCs in the presence or absence of SNCG were investigated by FACS analysis, ELISA, and real-time PCR. The ability of SNCG-treated DCs to influence T cells was also examined by coculturing with T cells. The treatment of DCs with SNCG protein inhibited the surface expression of the co-stimulatory molecules CD40 and CD86, and decreased the mRNA levels of pro-inflammatory cytokines. The SNCG-treated DCs inhibited T-cell proliferation slightly, but distinctively increased the population of regulatory T cells. In addition, the production of TGF-β from T cells was significantly increased when they were cocultured with SNCG-treated DCs. Taken together, these results demonstrate that tumor-derived SNCG contributes to immunosuppressive effects via the inhibition of DC differentiation and activation, thus making it a potential target for cancer treatment.
Figures


References
-
- Begg AC , Stewart FA , Vens C . Strategies to improve radiotherapy with targeted drugs. Nat Rev Cancer 2011; 11: 239–253. - PubMed
-
- Eriksson D , Stigbrand T . Radiation-induced cell death mechanisms. Tumour Biol 2010; 31: 363–372. - PubMed
-
- Buller S . Lasting attachments. Nursing Times 1989; 85: 36–37. - PubMed
-
- Ding Y , Yan Q , Ruan JW , Zhang YQ , Li WJ , Zeng X et al. Bone marrow mesenchymal stem cells and electroacupuncture downregulate the inhibitor molecules and promote the axonal regeneration in the transected spinal cord of rats. Cell Transplant 2011; 20: 475–491. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

