Pro-survival function of MEF2 in cardiomyocytes is enhanced by β-blockers
- PMID: 27551452
- PMCID: PMC4979494
- DOI: 10.1038/cddiscovery.2015.19
Pro-survival function of MEF2 in cardiomyocytes is enhanced by β-blockers
Abstract
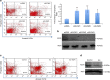
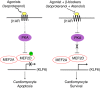
β1-Adrenergic receptor (β1-AR) stimulation increases apoptosis in cardiomyocytes through activation of cAMP/protein kinase A (PKA) signaling. The myocyte enhancer factor 2 (MEF2) proteins function as important regulators of myocardial gene expression. Previously, we reported that PKA signaling directly represses MEF2 activity. We determined whether (a) MEF2 has a pro-survival function in cardiomyocytes, and (b) whether β-adrenergic/PKA signaling modulates MEF2 function in cardiomyocytes. Initially, we observed that siRNA-mediated gene silencing of MEF2 induces cardiomyocyte apoptosis as indicated by flow cytometry. β1-AR activation by isoproterenol represses MEF2 activity and promotes apoptosis in cultured neonatal cardiomyocytes. Importantly, β1-AR mediated apoptosis was abrogated in cardiomyocytes expressing a PKA-resistant form of MEF2D (S121/190A). We also observed that a β1-blocker, Atenolol, antagonizes isoproterenol-induced apoptosis while concomitantly enhancing MEF2 transcriptional activity. β-AR stimulation modulated MEF2 cellular localization in cardiomyocytes and this effect was reversed by β-blocker treatment. Furthermore, Kruppel-like factor 6, a MEF2 target gene in the heart, functions as a downstream pro-survival factor in cardiomyocytes. Collectively, these data indicate that (a) MEF2 has an important pro-survival role in cardiomyocytes, and (b) β-adrenergic signaling antagonizes the pro-survival function of MEF2 in cardiomyocytes and β-blockers promote it. These observations have important clinical implications that may contribute to novel strategies for preventing cardiomyocyte apoptosis associated with heart pathology.
Figures

Similar articles
-
Suppression of a MEF2-KLF6 survival pathway by PKA signaling promotes apoptosis in embryonic hippocampal neurons.J Neurosci. 2012 Feb 22;32(8):2790-803. doi: 10.1523/JNEUROSCI.3609-11.2012. J Neurosci. 2012. PMID: 22357862 Free PMC article.
-
cAMP-mediated beta-adrenergic signaling negatively regulates Gq-coupled receptor-mediated fetal gene response in cardiomyocytes.J Mol Cell Cardiol. 2008 Dec;45(6):761-9. doi: 10.1016/j.yjmcc.2008.09.120. Epub 2008 Sep 25. J Mol Cell Cardiol. 2008. PMID: 18851973
-
β₁-adrenoceptor stimulation promotes LPS-induced cardiomyocyte apoptosis through activating PKA and enhancing CaMKII and IκBα phosphorylation.Crit Care. 2015 Mar 9;19(1):76. doi: 10.1186/s13054-015-0820-1. Crit Care. 2015. PMID: 25887954 Free PMC article.
-
The control of cardiomyocyte apoptosis via the beta-adrenergic signaling pathways.Arch Mal Coeur Vaiss. 2005 Mar;98(3):236-41. Arch Mal Coeur Vaiss. 2005. PMID: 15816327 Review.
-
Distinct beta-adrenergic receptor subtype signaling in the heart and their pathophysiological relevance.Sheng Li Xue Bao. 2004 Feb 25;56(1):1-15. Sheng Li Xue Bao. 2004. PMID: 14985822 Review.
Cited by
-
miR-410 and miR-495 Are Dynamically Regulated in Diverse Cardiomyopathies and Their Inhibition Attenuates Pathological Hypertrophy.PLoS One. 2016 Mar 21;11(3):e0151515. doi: 10.1371/journal.pone.0151515. eCollection 2016. PLoS One. 2016. PMID: 26999812 Free PMC article.
-
The MEF2A transcription factor interactome in cardiomyocytes.Cell Death Dis. 2023 Apr 5;14(4):240. doi: 10.1038/s41419-023-05665-8. Cell Death Dis. 2023. PMID: 37019881 Free PMC article.
-
Heart Failure and MEF2 Transcriptome Dynamics in Response to β-Blockers.Sci Rep. 2017 Jun 30;7(1):4476. doi: 10.1038/s41598-017-04762-x. Sci Rep. 2017. PMID: 28667250 Free PMC article.
-
Restorative Effects of Synbiotics on Colonic Ultrastructure and Oxidative Stress in Dogs with Chronic Enteropathy.Antioxidants (Basel). 2025 Jun 13;14(6):727. doi: 10.3390/antiox14060727. Antioxidants (Basel). 2025. PMID: 40563358 Free PMC article.
-
β-blockers and metabolic modulation: unraveling the complex interplay with glucose metabolism, inflammation and oxidative stress.Front Pharmacol. 2024 Dec 20;15:1489657. doi: 10.3389/fphar.2024.1489657. eCollection 2024. Front Pharmacol. 2024. PMID: 39759452 Free PMC article. Review.
References
-
- Dorn GW , Molkentin JD . Manipulating cardiac contractility in heart failure—Data from mice and men. Circulation 2004; 109: 150–158. - PubMed
-
- Iwai-Kanai E , Hasegawa K , Araki M , Kakita T , Morimoto T , Sasayama S . alpha- and beta-Adrenergic pathways differentially regulate cell type-specific apoptosis in rat cardiac myocytes. Circulation 1999; 100: 305–311. - PubMed
-
- Zaugg M , Xu WM , Lucchinetti E , Shafiq SA , Jamali NZ , Siddiqui MAQ . Beta-adrenergic receptor subtypes differentially affect apoptosis in adult rat ventricular myocytes. Circulation 2000; 102: 344–350. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

