Exposure to Endosulfan can result in male infertility due to testicular atrophy and reduced sperm count
- PMID: 27551453
- PMCID: PMC4979443
- DOI: 10.1038/cddiscovery.2015.20
Exposure to Endosulfan can result in male infertility due to testicular atrophy and reduced sperm count
Abstract
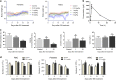
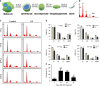
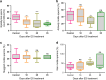
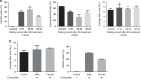
Endosulfan (ES) is a widely used organochlorine pesticide and is speculated to be detrimental to human health. However, very little is known about mechanism of its genotoxicity. Using mouse model system, we show that exposure to ES affected physiology and cellular architecture of organs and tissues. Among all organs, damage to testes was extensive and it resulted in death of different testicular-cell populations. We find that the damage in testes resulted in qualitative and quantitative defects during spermatogenesis in a time-dependent manner, increasing epididymal reactive oxygen species levels, affecting sperm chromatin integrity. This further culminated in reduced number of epididymal sperms and actively motile sperms. Finally, we show that ES exposure affected fertility in male but not in female mice. Therefore, we demonstrate that ES exerts pathophysiological changes in mice, induces testicular atrophy, affects spermatogenesis, reduces quantity and vigour of epididymal sperm and leads to infertility in males.
Figures



Similar articles
-
The influence of hormonal treatment with beta-human chorionic gonadotropin for cryptorchidism on future fertility in rats.J Pediatr Urol. 2015 Apr;11(2):92.e1-4. doi: 10.1016/j.jpurol.2014.12.011. Epub 2015 Mar 10. J Pediatr Urol. 2015. PMID: 25819376
-
Induction of DNA damage and erroneous repair can explain genomic instability caused by endosulfan.Carcinogenesis. 2016 Oct;37(10):929-40. doi: 10.1093/carcin/bgw081. Epub 2016 Aug 4. Carcinogenesis. 2016. PMID: 27492056
-
NTP technical report on the toxicity studies of Dibutyl Phthalate (CAS No. 84-74-2) Administered in Feed to F344/N Rats and B6C3F1 Mice.Toxic Rep Ser. 1995 Apr;30:1-G5. Toxic Rep Ser. 1995. PMID: 12209194
-
Review: Testicular vascular cone development and its association with scrotal thermoregulation, semen quality and sperm production in bulls.Animal. 2018 Jun;12(s1):s133-s141. doi: 10.1017/S1751731118001167. Animal. 2018. PMID: 29882506 Review.
-
Occupational exposure to pesticides and consequences on male semen and fertility: a review.Toxicol Lett. 2014 Oct 15;230(2):146-56. doi: 10.1016/j.toxlet.2014.01.029. Epub 2014 Jan 30. Toxicol Lett. 2014. PMID: 24487096 Review.
Cited by
-
Block Copolymer Encapsulation of Disarib, an Inhibitor of BCL2 for Improved Chemotherapeutic Potential.ACS Omega. 2023 Oct 19;8(43):40729-40740. doi: 10.1021/acsomega.3c05802. eCollection 2023 Oct 31. ACS Omega. 2023. PMID: 37929147 Free PMC article.
-
Protective effects of lupeol on pesticides induced testicular and oxidative damage of male rats.J Mol Histol. 2025 May 8;56(3):151. doi: 10.1007/s10735-025-10425-3. J Mol Histol. 2025. PMID: 40341998 Free PMC article.
-
Acute exposure to organophosphorus pesticide metabolites compromises buffalo sperm function and impairs fertility.Sci Rep. 2023 Jun 5;13(1):9102. doi: 10.1038/s41598-023-35541-6. Sci Rep. 2023. PMID: 37277402 Free PMC article.
-
Endosulfan induces reproductive & genotoxic effect in male and female Swiss albino mice.Lab Anim Res. 2024 May 22;40(1):22. doi: 10.1186/s42826-024-00208-4. Lab Anim Res. 2024. PMID: 38773665 Free PMC article.
-
Xeno-Estrogenic Pesticides and the Risk of Related Human Cancers.J Xenobiot. 2022 Nov 21;12(4):344-355. doi: 10.3390/jox12040024. J Xenobiot. 2022. PMID: 36412768 Free PMC article. Review.
References
-
- Fenner K , Canonica S , Wackett LP , Elsner M . Evaluating pesticide degradation in the environment: blind spots and emerging opportunities. Science 2013; 341: 752–758. - PubMed
-
- Ali U , Syed JH , Malik RN , Katsoyiannis A , Li J , Zhang G et al. Organochlorine pesticides (OCPs) in South Asian region: a review. Sci Total Environ 2014; 476-477: 705–717. - PubMed
-
- Sanpera C , Ruiz X , Llorente GA , Jover L , Jabeen R . Persistent organochlorine compounds in sediment and biota from the Haleji lake: a wildlife sanctuary in South Pakistan. Bull Environ Contam Toxicol 2002; 68: 237–244. - PubMed
-
- Carrera G , Fernandez P , Grimalt JO , Ventura M , Camarero L , Catalan J et al. Atmospherc deposition of organochlorine compounds to remote high mountain lakes of Europe. Environ Sci Technol 2002; 36: 2581–2588. - PubMed
-
- Pozo K , Harner T , Wania F , Muir DC , Jones KC , Barrie LA . Toward a global network for persistent organic pollutants in air: results from the GAPS study. Environ Sci Technol 2006; 40: 4867–4873. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

