Heme-based catalytic properties of human serum albumin
- PMID: 27551458
- PMCID: PMC4991842
- DOI: 10.1038/cddiscovery.2015.25
Heme-based catalytic properties of human serum albumin
Abstract
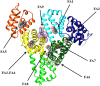
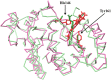
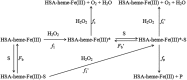
Human serum albumin (HSA): (i) controls the plasma oncotic pressure, (ii) modulates fluid distribution between the body compartments, (iii) represents the depot and carrier of endogenous and exogenous compounds, (iv) increases the apparent solubility and lifetime of hydrophobic compounds, (v) affects pharmacokinetics of many drugs, (vi) inactivates toxic compounds, (vii) induces chemical modifications of some ligands, (viii) displays antioxidant properties, and (ix) shows enzymatic properties. Under physiological and pathological conditions, HSA has a pivotal role in heme scavenging transferring the metal-macrocycle from high- and low-density lipoproteins to hemopexin, thus acquiring globin-like reactivity. Here, the heme-based catalytic properties of HSA are reviewed and the structural bases of drug-dependent allosteric regulation are highlighted.
Figures
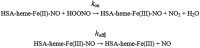
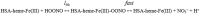
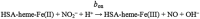

Similar articles
-
Drugs modulate allosterically heme-Fe-recognition by human serum albumin and heme-fe-mediated reactivity.Curr Pharm Des. 2015;21(14):1837-47. doi: 10.2174/1381612821666150302114430. Curr Pharm Des. 2015. PMID: 25732555 Review.
-
Serum Albumin: A Multifaced Enzyme.Int J Mol Sci. 2021 Sep 18;22(18):10086. doi: 10.3390/ijms221810086. Int J Mol Sci. 2021. PMID: 34576249 Free PMC article. Review.
-
Human serum albumin: from bench to bedside.Mol Aspects Med. 2012 Jun;33(3):209-90. doi: 10.1016/j.mam.2011.12.002. Epub 2011 Dec 30. Mol Aspects Med. 2012. PMID: 22230555 Review.
-
Heme Scavenging and Delivery: The Role of Human Serum Albumin.Biomolecules. 2023 Mar 22;13(3):575. doi: 10.3390/biom13030575. Biomolecules. 2023. PMID: 36979511 Free PMC article. Review.
-
Structural Basis of Drug Recognition by Human Serum Albumin.Curr Med Chem. 2020;27(30):4907-4931. doi: 10.2174/0929867326666190320105316. Curr Med Chem. 2020. PMID: 30894098 Review.
Cited by
-
Thiol catalyzed formation of NO-ferroheme regulates canonical intravascular NO signaling.Res Sq [Preprint]. 2023 Jan 20:rs.3.rs-2402224. doi: 10.21203/rs.3.rs-2402224/v1. Res Sq. 2023. Update in: Nat Chem Biol. 2023 Oct;19(10):1256-1266. doi: 10.1038/s41589-023-01413-3. PMID: 36711928 Free PMC article. Updated. Preprint.
-
Neutrophil-mediated oxidative stress and albumin structural damage predict COVID-19-associated mortality.Elife. 2021 Nov 25;10:e69417. doi: 10.7554/eLife.69417. Elife. 2021. PMID: 34821549 Free PMC article.
-
Ligand-Based Regulation of Dynamics and Reactivity of Hemoproteins.Biomolecules. 2023 Apr 17;13(4):683. doi: 10.3390/biom13040683. Biomolecules. 2023. PMID: 37189430 Free PMC article. Review.
-
Thiol-catalyzed formation of NO-ferroheme regulates intravascular NO signaling.Nat Chem Biol. 2023 Oct;19(10):1256-1266. doi: 10.1038/s41589-023-01413-3. Epub 2023 Sep 14. Nat Chem Biol. 2023. PMID: 37710075 Free PMC article.
-
Multi-wavelength analytical ultracentrifugation of human serum albumin complexed with porphyrin.Eur Biophys J. 2018 Oct;47(7):789-797. doi: 10.1007/s00249-018-1301-7. Epub 2018 Apr 19. Eur Biophys J. 2018. PMID: 29675648 Free PMC article.
References
-
- Peters T Jr . All About Albumin: Biochemistry, Genetics and Medical Applications. Academic Press: San Diego, CA, USA and London, UK, 1996.
-
- Fanali G , di Masi A , Trezza V , Marino M , Fasano M , Ascenzi P . Human serum albumin: from bench to bedside. Mol Aspects Med 2012; 33: 209–290. - PubMed
-
- Miller YI , Shaklai N . Kinetics of hemin distribution in plasma reveals its role in lipoprotein oxidation. Biochim Biophys Acta 1999; 1454: 153–164. - PubMed
-
- Ascenzi P , Bocedi A , Visca P , Altruda F , Tolosano E , Beringhelli T et al. Hemoglobin and heme scavenging. IUBMB Life 2005; 57: 749–759. - PubMed
-
- Ryter SW , Tyrrell RM . The heme synthesis and degradation pathways: role in oxidant sensitivity. Heme oxygenase has both pro- and antioxidant properties. Free Radic Biol Med 2000; 28: 289–309. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

