Gingival fibroblasts resist apoptosis in response to oxidative stress in a model of periodontal diseases
- PMID: 27551475
- PMCID: PMC4979524
- DOI: 10.1038/cddiscovery.2015.46
Gingival fibroblasts resist apoptosis in response to oxidative stress in a model of periodontal diseases
Abstract
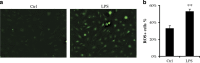
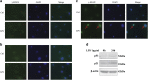
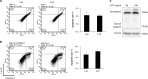
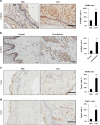
Periodontal diseases are classified as inflammation affecting the supporting tissue of teeth, which eventually leads to tooth loss. Mild reversible gingivitis and severe irreversible periodontitis are the most common periodontal diseases. Periodontal pathogens initiate the diseases. The bacterial toxin, lipopolysaccharide (LPS), triggers the inflammatory response and leads to oxidative stress. However, the progress of oxidative stress in periodontal diseases is unknown. The purpose of this study is to examine oxidative stress and cell damage in gingivitis and periodontitis. Our results showed that LPS increases reactive oxygen species (ROS) accumulation in gingival fibroblast (GF). However, oxidative stress resulting from excessive ROS did not influence DNA damage and cell apoptosis within 24 h. The mechanism may be related to the increased expression of DNA repair genes, Ogg1, Neil1 and Rad50. Detection of apoptosis-related proteins also showed anti-apoptotic effects and pro-apoptotic effects were balanced. The earliest damage appeared in DNA when increased γH2AX, an early biomarker for DNA damage, was detected in the LPS group after 48 h. Later, when recurrent inflammation persisted, 8-OHdG, a biomarker for oxidative stress was much higher in periodontitis model compared to the control in vivo. Staining of 8-OHdG in human periodontitis specimens confirmed the results. Furthermore, TUNEL staining of apoptotic cells indicated that the periodontitis model induced more cell apoptosis in gingival tissue. This suggested GF could resist early and acute inflammation (gingivitis), which was regarded as reversible, but recurrent and chronic inflammation (periodontitis) led to permanent cell damage and death.
Figures
Similar articles
-
Anti-inflammatory, anti-osteoclastic, and antioxidant activities of genistein protect against alveolar bone loss and periodontal tissue degradation in a mouse model of periodontitis.J Biomed Mater Res A. 2017 Sep;105(9):2510-2521. doi: 10.1002/jbm.a.36109. Epub 2017 Jun 6. J Biomed Mater Res A. 2017. PMID: 28509410
-
New evidence of premature oxidative DNA damage: mitochondrial DNA deletion in gingival tissue of patients with periodontitis.J Periodontol. 2006 Nov;77(11):1894-900. doi: 10.1902/jop.2006.060108. J Periodontol. 2006. PMID: 17076616
-
Lidocaine Prevents Oxidative Stress-Induced Endothelial Dysfunction of the Systemic Artery in Rats With Intermittent Periodontal Inflammation.Anesth Analg. 2017 Jun;124(6):2054-2062. doi: 10.1213/ANE.0000000000002102. Anesth Analg. 2017. PMID: 28525515
-
Pathways that Regulate ROS Scavenging Enzymes, and Their Role in Defense Against Tissue Destruction in Periodontitis.Front Physiol. 2017 May 30;8:351. doi: 10.3389/fphys.2017.00351. eCollection 2017. Front Physiol. 2017. PMID: 28611683 Free PMC article. Review.
-
Role of oxidative stress in the relationship between periodontitis and systemic diseases.Front Physiol. 2023 Jul 12;14:1210449. doi: 10.3389/fphys.2023.1210449. eCollection 2023. Front Physiol. 2023. PMID: 37501927 Free PMC article. Review.
Cited by
-
Characterization of the foreign body response of titanium implants modified with polyphenolic coatings.J Biomed Mater Res A. 2022 Jul;110(7):1341-1355. doi: 10.1002/jbm.a.37377. Epub 2022 Feb 26. J Biomed Mater Res A. 2022. PMID: 35218127 Free PMC article.
-
Periodontal Disease: The Good, The Bad, and The Unknown.Front Cell Infect Microbiol. 2021 Dec 7;11:766944. doi: 10.3389/fcimb.2021.766944. eCollection 2021. Front Cell Infect Microbiol. 2021. PMID: 34950607 Free PMC article. Review.
-
LPS from P. gingivalis Negatively Alters Gingival Cell Mitochondrial Bioenergetics.Int J Dent. 2017;2017:2697210. doi: 10.1155/2017/2697210. Epub 2017 May 16. Int J Dent. 2017. PMID: 28592970 Free PMC article.
-
Typhoid toxin exhausts the RPA response to DNA replication stress driving senescence and Salmonella infection.Nat Commun. 2019 Sep 6;10(1):4040. doi: 10.1038/s41467-019-12064-1. Nat Commun. 2019. PMID: 31492859 Free PMC article.
-
Investigating the Origins of Toxic Response in TiO₂ Nanoparticle-Treated Cells.Nanomaterials (Basel). 2017 Apr 11;7(4):83. doi: 10.3390/nano7040083. Nanomaterials (Basel). 2017. PMID: 28398241 Free PMC article.
References
-
- Page RC , Offenbacher S , Schroeder HE , Seymour GJ , Kornman KS . Advances in the pathogenesis of periodontitis: summary of developments, clinical implications and future directions. Periodontol 2000 1997; 14: 216–248. - PubMed
-
- Gorąca A , Huk-Kolega H , Kleniewska P , Piechota-Polańczyk A , Skibska B . Effects of lipoic acid on spleen oxidative stress after LPS administration. Pharmacol Rep 2013; 65: 179–186. - PubMed
-
- Melo ES , Barbeiro HV , Ariga S , Goloubkova T , Curi R , Valasco IT et al. Immune cells and oxidative stress in the endotoxin tolerance mouse model. Braz J Med Biol Res 2010; 43: 57–67. - PubMed
-
- Sanikidze TV , Tkhilava NG , Papava MB , Datunashvili IV , Gongadze MT , Gamrekelashvili DD et al. Role of free nitrogen and oxygen radical in the pathogenesis of lipopolysaccharide-induced endotoxemia. Bull Exp Biol Med 2006; 141: 211–215. - PubMed
-
- Bykov I , Ylipaasto P , Erola L , Lindros KO . Phagocytosis and LPS stimulated production of cytokines and prostaglandin E2 is different in Kupffer cells isolated from the periportal or perivenous liver region. Scand J Gastroenterol 2003; 38: 1256–1261. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

