Cigarette smoke-induced cell death of a spermatocyte cell line can be prevented by inactivating the Aryl hydrocarbon receptor
- PMID: 27551479
- PMCID: PMC4979462
- DOI: 10.1038/cddiscovery.2015.50
Cigarette smoke-induced cell death of a spermatocyte cell line can be prevented by inactivating the Aryl hydrocarbon receptor
Abstract
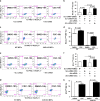
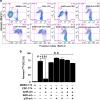
Cigarette smoke exposure causes germ cell death during spermatogenesis. Our earlier studies demonstrated that cigarette smoke condensate (CSC) causes spermatocyte cell death in vivo and growth arrest of the mouse spermatocyte cell line (GC-2spd(ts)) in vitro via the aryl hydrocarbon receptor (AHR). We hypothesize here that inactivation of AHR could prevent the CSC-induced cell death in spermatocytes. We demonstrate that CSC exposure generates oxidative stress, which differentially regulates mitochondrial apoptosis in GC-2spd(ts) and wild type (WT) and AHR knockout (AHR-KO) mouse embryonic fibroblasts (MEFs). SiRNA-mediated silencing of Ahr augments the extent of CSC-mediated cellular damage while complementing the AHR-knockout condition. Pharmacological inhibition using the AHR-antagonist (CH223191) modulates the CSC-altered expression of apoptotic proteins and significantly abrogates DNA fragmentation though the cleavage of PARP appears AHR independent. Pretreatment with CH223191 at concentrations above 50 μM significantly prevents the CSC-induced activation of caspase-3/7 and externalization of phosphatidylserine in the plasma membrane. However, MAPK inhibitors alone or together with CH223191 could not prevent the membrane damage upon CSC addition and the caspase-3/7 activation and membrane damage in AHR-deficient MEF indicates the interplay of multiple cell signaling and cytoprotective ability of AHR. Thus the data obtained on one hand signifies the protective role of AHR in maintaining normal cellular homeostasis and the other, could be a potential prophylactic therapeutic target to promote cell survival and growth under cigarette smoke exposed environment by receptor antagonism via CH223191-like mechanism. Antagonist-mediated inactivation of the aryl hydrocarbon receptor blocks downstream events leading to cigarette smoke-induced cell death of a spermatocyte cell line.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





Similar articles
-
Paternal smoking and germ cell death: A mechanistic link to the effects of cigarette smoke on spermatogenesis and possible long-term sequelae in offspring.Mol Cell Endocrinol. 2016 Nov 5;435:85-93. doi: 10.1016/j.mce.2016.07.015. Epub 2016 Jul 14. Mol Cell Endocrinol. 2016. PMID: 27424142 Free PMC article. Review.
-
Cigarette smoke-induced cell cycle arrest in spermatocytes [GC-2spd(ts)] is mediated through crosstalk between Ahr-Nrf2 pathway and MAPK signaling.J Mol Cell Biol. 2015 Feb;7(1):73-87. doi: 10.1093/jmcb/mju049. Epub 2014 Dec 29. J Mol Cell Biol. 2015. PMID: 25548370
-
Modulation of cell cycle progression in the spermatocyte cell line [GC-2spd(ts) Cell-Line] by cigarette smoke condensate (CSC) via arylhydrocarbon receptor-nuclear factor erythroid 2-related factor 2 (Ahr-Nrf2) pathway.Biol Reprod. 2014 Jan 16;90(1):9. doi: 10.1095/biolreprod.113.113225. Print 2014 Jan. Biol Reprod. 2014. PMID: 24258214
-
Cigarette smoke condensate induces aryl hydrocarbon receptor-dependent changes in gene expression in spermatocytes.Reprod Toxicol. 2012 Dec;34(4):665-76. doi: 10.1016/j.reprotox.2012.10.005. Epub 2012 Oct 13. Reprod Toxicol. 2012. PMID: 23069111
-
Genetic ablation of the aryl hydrocarbon receptor causes cigarette smoke-induced mitochondrial dysfunction and apoptosis.J Biol Chem. 2011 Dec 16;286(50):43214-28. doi: 10.1074/jbc.M111.258764. Epub 2011 Oct 7. J Biol Chem. 2011. PMID: 21984831 Free PMC article.
Cited by
-
Paternal smoking and germ cell death: A mechanistic link to the effects of cigarette smoke on spermatogenesis and possible long-term sequelae in offspring.Mol Cell Endocrinol. 2016 Nov 5;435:85-93. doi: 10.1016/j.mce.2016.07.015. Epub 2016 Jul 14. Mol Cell Endocrinol. 2016. PMID: 27424142 Free PMC article. Review.
-
The effect of hydroalcoholic extract of Cichorium intybus leaf on aryl hydrocarbon receptor expression in the testis of Wistar rats exposed to cigarette smoke.Avicenna J Phytomed. 2023 Jan-Feb;13(1):58-69. doi: 10.22038/AJP.2022.21307. Avicenna J Phytomed. 2023. PMID: 36698732 Free PMC article.
-
Alternative Oxidase Attenuates Cigarette Smoke-induced Lung Dysfunction and Tissue Damage.Am J Respir Cell Mol Biol. 2019 May;60(5):515-522. doi: 10.1165/rcmb.2018-0261OC. Am J Respir Cell Mol Biol. 2019. PMID: 30339461 Free PMC article.
-
Elevated Cellular Oxidative Stress in Circulating Immune Cells in Otherwise Healthy Young People Who Use Electronic Cigarettes in a Cross-Sectional Single-Center Study: Implications for Future Cardiovascular Risk.J Am Heart Assoc. 2020 Sep 15;9(18):e016983. doi: 10.1161/JAHA.120.016983. Epub 2020 Sep 8. J Am Heart Assoc. 2020. PMID: 32896211 Free PMC article.
-
Preventing germ cell death by inactivating aryl hydrocarbon receptor (AHR).Cell Death Dis. 2016 Feb 25;7(2):e2116. doi: 10.1038/cddis.2016.20. Cell Death Dis. 2016. PMID: 26913606 Free PMC article. No abstract available.
References
-
- Smoking and infertility: a committee opinion. The Practice Committee of the American Society for Reproductive Medicine. Fertil Steril 2012; 98: 1400–1406. - PubMed
-
- Rodgman A , Perfetti TA . The Chemical Components of Tobacco and Tobacco Smoke. CRC Press, Taylor & Francis Group: Boca Raton, FL, USA, 2009.
-
- Ramlau-Hansen CH , Thulstrup AM , Aggerholm AS , Jensen MS , Toft G , Bonde JP . Is smoking a risk factor for decreased semen quality? A cross-sectional analysis. Hum Reprod 2007; 22: 188–196. - PubMed
-
- Viczian M . The effect of cigarette smoke inhalation on spermatogenesis in rats. Experientia 1968; 24: 511–513. - PubMed
-
- Georgellis A , Montelius J , Rydström J . Evidence for a free-radical-dependent metabolism of 7,12-dimethylbenz[a]anthracene in rat testis. Toxicol Appl Pharmacol 1987; 87: 141–154. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

