Resveratrol chemosensitizes HER-2-overexpressing breast cancer cells to docetaxel chemoresistance by inhibiting docetaxel-mediated activation of HER-2-Akt axis
- PMID: 27551486
- PMCID: PMC4979566
- DOI: 10.1038/cddiscovery.2015.61
Resveratrol chemosensitizes HER-2-overexpressing breast cancer cells to docetaxel chemoresistance by inhibiting docetaxel-mediated activation of HER-2-Akt axis
Abstract
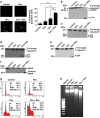
As breast cancer cells often develop chemoresistance, better therapeutic options are in search to circumvent it. Here we demonstrate that human epidermal growth factor receptor-2 (HER-2)-overexpressing breast cancer cells resist docetaxel-induced cytotoxicity by upregulating HER-2 and its activity downstream, through Akt and mitogen-activated protein kinase (MAPK) pathways. We observed that introducing resveratrol as a chemosensitizer in docetaxel chemotherapy blocks upregulation and activation of HER-2 in addition to blocking downstream signaling pathways such as Akt. Resveratrol and docetaxel combination results in the synergistic induction of cell death in HER-2-overexpressing SK-BR-3 cells, whereas introduction of wild-type HER-2 in MDA-MD-231 cells increased the resistance to docetaxel. Dominant-negative HER-2 sensitizes SK-BR-3 cells to docetaxel. Our study identified a new synergistic therapeutic combination that targets HER-2-induced breast cancer resistance and might help to overcome therapeutic resistance during breast cancer therapy. The synergism of docetaxel and resveratrol was maximum in SK-BR-3, which is unique among the cell lines studied, due to its high expression status of HER-2, a receptor known to dictate the signaling environment of breast cancer cells. Docetaxel could further induce HER-2 activity in these cells, which was downregulated on resveratrol treatment. Transfection of DN-HER-2 in SK-BR-3 cells inhibits the synergism as the transfection itself sensitizes these cells to docetaxel, leaving no role for resveratrol, whereas ectopic expression of HER-2 introduces the synergism in MDA-MB-231, the triple-negative cell line, in which the synergism was minimum, attesting the crucial role of HER-2 in suppressing the sensitivity to docetaxel. Single-agent docetaxel induced HER-2-mediated resistance to cell death, which was blocked by resveratrol. Resveratrol also downregulated docetaxel-induced activation of MAPK and Akt, survival signaling pathways downstream of HER-2. In short, this study, for the first time, establishes the role of HER-2-Akt signaling axis in regulating the synergistic effect of docetaxel and resveratrol in breast cancer cells overexpressing HER-2.
Figures




Similar articles
-
Inhibition of tumor-associated fatty acid synthase hyperactivity induces synergistic chemosensitization of HER -2/ neu -overexpressing human breast cancer cells to docetaxel (taxotere).Breast Cancer Res Treat. 2004 Mar;84(2):183-95. doi: 10.1023/B:BREA.0000018409.59448.60. Breast Cancer Res Treat. 2004. PMID: 14999148
-
Pharmacological and small interference RNA-mediated inhibition of breast cancer-associated fatty acid synthase (oncogenic antigen-519) synergistically enhances Taxol (paclitaxel)-induced cytotoxicity.Int J Cancer. 2005 May 20;115(1):19-35. doi: 10.1002/ijc.20754. Int J Cancer. 2005. PMID: 15657900
-
Omega-6 polyunsaturated fatty acid gamma-linolenic acid (18:3n-6) enhances docetaxel (Taxotere) cytotoxicity in human breast carcinoma cells: Relationship to lipid peroxidation and HER-2/neu expression.Oncol Rep. 2004 Jun;11(6):1241-52. Oncol Rep. 2004. PMID: 15138562
-
The MEK/MAPK pathway is involved in the resistance of breast cancer cells to the EGFR tyrosine kinase inhibitor gefitinib.J Cell Physiol. 2006 May;207(2):420-7. doi: 10.1002/jcp.20588. J Cell Physiol. 2006. PMID: 16419029
-
A Review of Resveratrol as a Potent Chemoprotective and Synergistic Agent in Cancer Chemotherapy.Front Pharmacol. 2019 Jan 9;9:1534. doi: 10.3389/fphar.2018.01534. eCollection 2018. Front Pharmacol. 2019. PMID: 30687096 Free PMC article. Review.
Cited by
-
Natural Small Molecules in Breast Cancer Treatment: Understandings from a Therapeutic Viewpoint.Molecules. 2022 Mar 27;27(7):2165. doi: 10.3390/molecules27072165. Molecules. 2022. PMID: 35408561 Free PMC article. Review.
-
Ginsenoside Rg5 Sensitizes Paclitaxel-Resistant Human Cervical-Adeno-Carcinoma Cells to Paclitaxel-And Enhances the Anticancer Effect of Paclitaxel.Genes (Basel). 2022 Jun 24;13(7):1142. doi: 10.3390/genes13071142. Genes (Basel). 2022. PMID: 35885925 Free PMC article.
-
Disease-associated regulation of gene expression by resveratrol: Special focus on the PI3K/AKT signaling pathway.Cancer Cell Int. 2022 Sep 30;22(1):298. doi: 10.1186/s12935-022-02719-3. Cancer Cell Int. 2022. PMID: 36180892 Free PMC article. Review.
-
Resveratrol As A Natural Regulator Of Autophagy For Prevention And Treatment Of Cancer.Onco Targets Ther. 2019 Oct 17;12:8601-8609. doi: 10.2147/OTT.S213043. eCollection 2019. Onco Targets Ther. 2019. PMID: 31802896 Free PMC article.
-
Phyto-therapeutics as anti-cancer agents in breast cancer: Pathway targeting and mechanistic elucidation.Saudi J Biol Sci. 2024 Mar;31(3):103935. doi: 10.1016/j.sjbs.2024.103935. Epub 2024 Jan 20. Saudi J Biol Sci. 2024. PMID: 38327657 Free PMC article. Review.
References
-
- Sui M , Zhang H , Fan W . The role of estrogen and estrogen receptors in chemoresistance. Curr Med Chem 2011; 18: 4674–4683. - PubMed
-
- Zhang W , Ding W , Chen Y , Feng M , Ouyang Y , Yu Y et al. Up-regulation of breast cancer resistance protein plays a role in HER2-mediated chemoresistance through PI3K/Akt and nuclear factor-kappa B signaling pathways in MCF7 breast cancer cells. Acta Biochim Biophys Sin (Shanghai) 2011; 43: 647–653. - PubMed
-
- Lumachi F , Luisetto G , Basso SM , Basso U , Brunello A , Camozzi V . Endocrine therapy of breast cancer. Curr Med Chem 2011; 18: 513–522. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

