Development of a lytic peptide derived from BH3-only proteins
- PMID: 27551502
- PMCID: PMC4979451
- DOI: 10.1038/cddiscovery.2016.8
Development of a lytic peptide derived from BH3-only proteins
Abstract
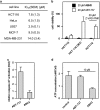
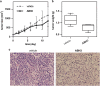
Despite great advances in cancer therapy, drug resistance is a difficult hurdle to overcome that requires development of anticancer agents with novel and effective modes of action. In a number of studies, lytic peptides have shown remarkable ability to eliminate cancer cells through a different way from traditional treatments. Lytic peptides are positively charged, amphiphilic, and are efficient at binding and disrupting the negatively charged cell membrane of cancer cells. In this study, we described the anticancer properties of a lytic peptide that was developed on the basis of the alignment of amphiphilic BH3 peptides. Our results demonstrated that the positive charge and conformation constraint were favourable for efficient cancer cell elimination. Artificial BCL-2 homology 3 peptides (ABH3) exhibited effective anticancer effects against a series of cancer cell lines in vitro and in HeLa human cervical tumour xenografts in vivo. ABH3 induced cell death in an apoptosis-independent manner through the lytic properties of the peptide that caused disruption of cell membrane. Our results showed that charge tuning and conformation constraining in a lytic peptide could be applied to optimise the anticancer activity of lytic peptides. These results also suggest that ABH3 may be a promising beginning for the development of additional lytic peptides as anticancer reagents.
Figures
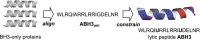


References
-
- Chen L , Tu Z , Voloshchuk N , Liang JF . Lytic peptides with improved stability and selectivity designed for cancer treatment. J Pharm Sci 2012; 101: 1508–1517. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

