Cytotoxic L-amino-acid oxidases from Amanita phalloides and Clitocybe geotropa induce caspase-dependent apoptosis
- PMID: 27551514
- PMCID: PMC4979486
- DOI: 10.1038/cddiscovery.2016.21
Cytotoxic L-amino-acid oxidases from Amanita phalloides and Clitocybe geotropa induce caspase-dependent apoptosis
Abstract
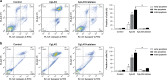
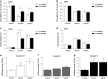
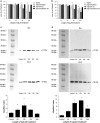
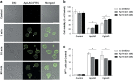
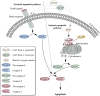
L-amino-acid oxidases (LAO) purified from fungi induce cell death in various mammalian cells including human tumor cell lines. The mechanism, however, remains poorly understood. In this study, we aimed to define a precise mechanism of cell death induced in Jurkat and MCF7 cancer cell lines by ApLAO and CgLAO, LAOs isolated from Amanita phalloides and Clitocybe geotropa, respectively. Cell death induced by both LAOs is shown to be concentration- and time-dependent, with higher toxic effects in Jurkat cells. LAO activity is required for the cytotoxicity. Detailed study on Jurkat cells further demonstrated that ApLAO and CgLAO both induce the intrinsic mitochondrial pathway of apoptosis, accompanied by a time-dependent depolarization of the mitochondrial membrane through the generation of reactive oxygen species. Treatment with the LAOs resulted in an increased ratio of the expression of proapoptotic Bax to that of antiapoptotic Bcl-2, subsequently leading to the activation of caspase-9 and -3. However, the pancaspase inhibitor, Z-VAD-FMK, did not completely abolish the cell death induced by either ApLAO or CgLAO, suggesting an alternative pathway for LAO-induced apoptosis. Indeed, caspase-8 activity in ApLAO- and CgLAO-treated cells was increased. Further, Fas/FasL (Fas ligand) antagonist caused a slight reduction in toxin-induced cell death, supporting the involvement of ApLAO and CgLAO in death-receptor-mediated apoptosis. These results thus provide new evidence that ApLAO and CgLAO induce apoptosis in Jurkat cells via both the intrinsic and extrinsic pathways, although the significantly higher increase of caspase-9 over caspase-8 activity suggests that it is the intrinsic pathway that is the predominant mode of ApLAO- and CgLAO-induced apoptosis.
Figures


References
-
- Fesik SW. Promoting apoptosis as a strategy for cancer drug discovery. Nat Rev Cancer 2005; 5: 876–885. - PubMed
-
- Walczak H, Krammer PH. The CD95 (APO-1/Fas) and the TRAIL (APO-2L) apoptosis systems. Exp Cell Res 2000; 256: 58–66. - PubMed
-
- Eldering E, Mackus WJ, Derks IA, Evers LM, Beuling E, Teeling P et al. Apoptosis via the B cell antigen receptor requires Bax translocation and involves mitochondrial depolarization, cytochrome C release, and caspase-9 activation. Eur J Immunol 2004; 34: 1950–1960. - PubMed
-
- Fulda S, Debatin KM. Extrinsic versus intrinsic apoptosis pathways in anticancer chemotherapy. Oncogene 2006; 25: 4798–4811. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

