Sleep recalibrates homeostatic and associative synaptic plasticity in the human cortex
- PMID: 27551934
- PMCID: PMC4996971
- DOI: 10.1038/ncomms12455
Sleep recalibrates homeostatic and associative synaptic plasticity in the human cortex
Abstract
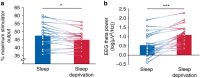
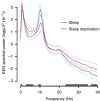
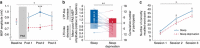
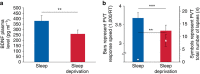
Sleep is ubiquitous in animals and humans, but its function remains to be further determined. The synaptic homeostasis hypothesis of sleep-wake regulation proposes a homeostatic increase in net synaptic strength and cortical excitability along with decreased inducibility of associative synaptic long-term potentiation (LTP) due to saturation after sleep deprivation. Here we use electrophysiological, behavioural and molecular indices to non-invasively study net synaptic strength and LTP-like plasticity in humans after sleep and sleep deprivation. We demonstrate indices of increased net synaptic strength (TMS intensity to elicit a predefined amplitude of motor-evoked potential and EEG theta activity) and decreased LTP-like plasticity (paired associative stimulation induced change in motor-evoked potential and memory formation) after sleep deprivation. Changes in plasma BDNF are identified as a potential mechanism. Our study indicates that sleep recalibrates homeostatic and associative synaptic plasticity, believed to be the neural basis for adaptive behaviour, in humans.
Conflict of interest statement
Competing financial interests: D.R. has received a consulting fee from Abbvie Germany. C.No. has received speaker honoraria from Servier and Roche. He is an investigator in multicentre clinical trials sponsored by Otsuka, Lundbeck, Roche and Forum Pharmaceuticals. He received research support from Lundbeck and the German Ministry of Research and Education. C.Ni. has received speaker honoraria from Servier and Vanda Pharmaceuticals. The remaining authors declare no competing financial interests.
Figures

References
-
- Turrigiano G. G. & Nelson S. B. Homeostatic plasticity in the developing nervous system. Nat. Rev. Neurosci. 5, 97–107 (2004). - PubMed
-
- Diekelmann S. & Born J. The memory function of sleep. Nat. Rev. Neurosci. 11, 114–126 (2010). - PubMed
-
- Tononi G. & Cirelli C. Sleep and synaptic homeostasis: a hypothesis. Brain Res. Bull. 62, 143–150 (2003). - PubMed
-
- Tononi G. & Cirelli C. Sleep function and synaptic homeostasis. Sleep Med. Rev. 10, 49–62 (2006). - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

