Vitamin A supplementation to prevent mortality and short- and long-term morbidity in very low birth weight infants
- PMID: 27552058
- PMCID: PMC7038719
- DOI: 10.1002/14651858.CD000501.pub4
Vitamin A supplementation to prevent mortality and short- and long-term morbidity in very low birth weight infants
Abstract
Background: Vitamin A is necessary for normal lung growth and the integrity of respiratory tract epithelial cells. Preterm infants have low vitamin A status at birth and this has been associated with an increased risk of developing chronic lung disease.
Objectives: To evaluate supplementation with vitamin A on the incidence of death or neonatal chronic lung disease and long-term neurodevelopmental disability in very low birth weight (VLBW) infants compared with a control (placebo or no supplementation), and to consider the effect of the supplementation route, dose, and timing.
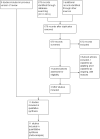
Search methods: For the original review and subsequent updates, we searched the Cochrane Central Register of Controlled Trials (CENTRAL, The Cochrane Library), MEDLINE, Science Citation Index, and the Oxford Database of Perinatal Trials. The reference lists of relevant trials, paediatric and nutrition journals, and conference abstracts and proceedings were handsearched up to 2010.For the 2016 update, we used the standard search strategy of the Cochrane Neonatal Review group to search the Cochrane Central Register of Controlled Trials (CENTRAL 2016, Issue 4), MEDLINE via PubMed (1 May 2016), EMBASE (1 May 2016), and CINAHL (1 May 2016). We also searched clinical trials' databases, conference proceedings, and the reference lists of retrieved articles for randomised controlled trials and quasi-randomised trials.
Selection criteria: Randomised controlled trials comparing vitamin A supplementation with a control (placebo or no supplementation) or other dosage regimens in VLBW infants (birth weight ≤ 1500 grams or less than 32 weeks' gestation).
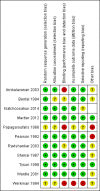
Data collection and analysis: Two review authors screened the search results, extracted data, and assessed the trials for risk of bias. Results were reported as risk ratios (RR), risk differences (RD), and number needed to treat to benefit (NNTB), all with 95% confidence intervals (CI). Trialists were contacted for additional data.
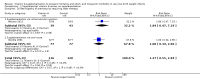
Main results: Eleven trials met the inclusion criteria. Ten trials (1460 infants) compared vitamin A supplementation with a control and one (120 infants) compared different regimens of vitamin A supplementation. Compared to the control group, vitamin A appeared to have a small benefit in reducing the risk of death or oxygen requirement at one month of age (typical RR 0.93, 95% CI 0.88 to 0.99; typical RD -0.05, 95% CI -0.10 to -0.01; NNTB 20, 95% CI 10 to 100; 6 studies, 1165 infants) and the risk of chronic lung disease (oxygen requirement) at 36 weeks' postmenstrual age (typical RR 0.87, 95% CI 0.77 to 0.99; typical RD -0.07, 95% CI -0.13 to -0.01; NNTB 11, 95% CI 6 to 100; 5 studies, 986 infants) (moderate-quality evidence). There was a marginal reduction of the combined outcome of death or chronic lung disease (typical RR 0.92, 95% CI 0.84 to 1.01; typical RD -0.05, 95% CI -0.11 to 0.01; 4 studies, 1089 infants). Neurodevelopmental assessment of 88% of the surviving infants in the largest trial showed no difference between the groups at 18 to 22 months of age, corrected for prematurity (low-quality evidence). There is no evidence to support different vitamin A dosing regimens. No adverse effects of vitamin A supplementation were reported, but it was noted that intramuscular injections of vitamin A were painful.
Authors' conclusions: Whether clinicians decide to utilise repeat intramuscular doses of vitamin A to prevent chronic lung disease may depend upon the local incidence of this outcome and the value attached to achieving a modest reduction in the outcome balanced against the lack of other proven benefits and the acceptability of the treatment. Information on long-term neurodevelopmental status suggests no evidence of either benefit or harm from the intervention.
Conflict of interest statement
None known.
Figures






































Update of
-
Vitamin A supplementation to prevent mortality and short- and long-term morbidity in very low birthweight infants.Cochrane Database Syst Rev. 2011 Oct 5;(10):CD000501. doi: 10.1002/14651858.CD000501.pub3. Cochrane Database Syst Rev. 2011. Update in: Cochrane Database Syst Rev. 2016 Aug 22;(8):CD000501. doi: 10.1002/14651858.CD000501.pub4. PMID: 21975731 Updated.
References
References to studies included in this review
Ambalavanan 2003 {published and unpublished data}
-
- Ambalavanan N, Wu TJ, Tyson JE, Kennedy KA, Roane C, Carlo WA. A comparison of three vitamin A dosing regimens in extremely‐low‐birth‐weight infants. Journal of Pediatrics 2003;142(6):656‐61. - PubMed
Bental 1994 {published and unpublished data}
-
- Bental RY, Cooper PA, Cummins RR, Sandler DL, Wainer S, Rotschild A. Vitamin A therapy ‐ effects on the incidence of bronchopulmonary dysplasia. South African Journal of Food Science and Nutrition 1994;6(4):141‐5.
-
- Bental RY, Cooper PA, Sandler D, Wainer S. The effects of vitamin A therapy on the incidence of chronic lung disease in premature infants [abstract]. Pediatric Research 1990;25:296A.
Kiatchoosakun 2014 {published data only}
-
- Kiatchoosakun P, Jirapradittha J, Panthongviriyakul MC, Khampitak T, Yongvanit P, Boonsiri P. Vitamin A supplementation for prevention of bronchopulmonary dysplasia in very‐low‐birth‐weight premature Thai infants: a randomized trial. Journal of the Medical Association of Thailand 2014;97(Suppl 10):S82‐8. [PUBMED: 25816542] - PubMed
Mactier 2012 {published data only}
-
- Mactier H, McCulloch DL, Hamilton R, Galloway P, Bradnam MS, Young D, et al. Vitamin A supplementation improves retinal function in infants at risk of retinopathy of prematurity. Journal of Pediatrics 2012;160(6):954‐9. - PubMed
Papagaroufalis 1988 {unpublished data only}
-
- Papagaroufalis C, Cairis M, Pantazatou E, Meghreli Ch, Xanthou M. A trial of vitamin A supplementation in infants susceptible to bronchopulmonary dysplasia [abstract]. Pediatric Research 1988;23:518A.
Pearson 1992 {published data only}
-
- Pearson E, Bose C, Snidow T, Ransom L, Young T, Bose G, et al. Trial of vitamin A supplementation in very low birth weight infants at risk for bronchopulmonary dysplasia. Journal of Pediatrics 1992;121(3):420‐7. - PubMed
Ravishankar 2003 {published and unpublished data}
-
- Nafday S, Ravishankar C, Green RS, Kamenir SA, Lorber R, Stacewicz‐Sapuntzakis M, et al. A trial of vitamin A therapy to facilitate ductal closure in premature infants [abstract]. Pediatric Research 2002;51:308A. - PubMed
-
- Ravishankar C, Nafday S, Green RS, Kamenir S, Lorber R, Stacewicz‐Sapuntzakis M, et al. A trial of vitamin A therapy to facilitate ductal closure in premature infants. Journal of Pediatrics 2003;143(5):644‐8. - PubMed
Shenai 1987 {published data only}
-
- Shenai JP, Kennedy KA, Chytil F, Stahlman MT. Clinical trial of vitamin A supplementation in infants susceptible to bronchopulmonary dysplasia. Journal of Pediatrics 1987;111(2):269‐77. - PubMed
Tyson 1999 {published data only}
-
- Ambalavanan N, Tyson JE, Kennedy KA, Hansen NI, Vohr BR, Wright LL, et al, National Institute of Child Health and Human Development Neonatal Research Network. Vitamin A supplementation for extremely low birth weight infants: outcome at 18 to 22 months. Pediatrics 2005;115(3):e249‐54. - PubMed
-
- Londhe VA, Nolen TL, Das A, Higgins RD, Tyson JE, Oh W, et al. Eunice Kennedy Shriver National Institute of Child Health and Human Development Neonatal Research Network. Vitamin A supplementation in extremely low‐birth‐weight infants: subgroup analysis in small‐for‐gestational‐age infants. American Journal of Perinatology 2013;30(9):771‐80. [DOI: 10.1055/s-0032-1333410] - DOI - PMC - PubMed
-
- Tyson JE, Ehrenkranz RA, Stoll BJ, Wright LL, Mele L, Kennedy KA, et al. Vitamin (Vit) A supplementation to increase survival without chronic lung disease (CLD) in extremely low birth weight (ELBW) infants: a 14‐center randomized trial [abstract]. Pediatric Research 1998;43:199A.
-
- Tyson JE, Wright LL, Oh W, Kennedy KA, Mele L, Ehrenkranz RA, et al. Vitamin A supplementation for extremely‐low‐birth‐weight infants. National Institute of Child Health and Human Development Neonatal Research Network. New England Journal of Medicine 1999;340(25):1962‐8. - PubMed
Wardle 2001 {published and unpublished data}
Werkman 1994 {published data only}
-
- Werkman SH, Peeples JM, Cooke RJ, Tolley EA, Carlson SE. Effect of vitamin A supplementation of intravenous lipids on early vitamin A intake and status of premature infants. American Journal of Clinical Nutriton 1994;59(3):586‐92. - PubMed
References to studies excluded from this review
Aranda 1992 {unpublished data only}
-
- Aranda JV, Akramoff L, Beharry K, Ling E, Papageorgiou A. Plasma concentrations of vitamin A and E during supplemental therapy in premature infants [abstract]. Pediatric Research 1992;31:57A.
Aurvag 2007 {published data only}
-
- Aurvåg AK, Henriksen C, Drevon CA, Iversen PO, Nakstad B. Improved vitamin A supplementation regimen for breastfed very low birth weight infant. Acta Pædiatrica 2007;96(9):1296‐302. - PubMed
Coutsoudis 1996 {published data only}
-
- Coutsoudis A, Adhikari M, Pillay K, Coovadia HM. Absorption of high‐dose enteral vitamin A in low‐birth‐weight neonates. South African Medical Journal 1996;86(10 Suppl):1337‐9. - PubMed
Coutsoudis 2000 {published data only}
-
- Coutsoudis A, Adhikari M, Pillay K, Kuhn L, Coovadia HM. Effect of vitamin A supplementation on morbidity of low‐birth‐weight neonates. South African Medical Journal 2000;90(7):730‐6. - PubMed
Koo 1993 {unpublished data only}
-
- Koo W, Krug‐Wispe S, Tsang R. Effect of differential enteral vitamin A intakes in very low birth weight infants [abstract]. Pediatric Research 1993;33:306A.
-
- Koo WW, Krug‐Wispe S, Succop P, Tsang RC, Neylan M. Effect of different vitamin A intakes on very‐low‐birth‐weight infants. American Journal of Clinical Nutrition 1995;62(6):1216‐20. - PubMed
Landman 1992 {published data only}
-
- Landman J, Sive A, Heese HD, Van der Elst C, Sacks R. Comparison of enteral and intramuscular vitamin A supplementation in preterm infants. Early Human Development 1992;30(2):163‐70. - PubMed
Longardt 2014 {published data only}
-
- Longardt AC, Schmiedchen B, Raila J, Schweigert FJ, Obladen M, Bührer C, et al. Characterization of the vitamin A transport in preterm infants after repeated high‐dose vitamin A injections. European Journal of Clinical Nutrition 2014;68(12):1300‐4. [PUBMED: 25315494] - PubMed
Robbins 1993 {published data only}
-
- Robbins ST, Fletcher AB. Early vs delayed vitamin A supplementation in very‐low‐birth‐weight infants. Journal of Parenteral and Enteral Nutrition 1993;17(3):220‐5. - PubMed
Rush 1994 {published data only}
-
- Rush MG, Shenai JP, Parker RA, Chytil F. Intramuscular versus enteral vitamin A supplementation in very low birth weight neonates. Journal of Pediatrics 1994;125(3):458‐62. - PubMed
Schmiedchen 2016 {published data only}
-
- Schmiedchen B, Longardt AC, Loui A, Bührer C, Raila J, Schweigert FJ. Effect of vitamin A supplementation on the urinary retinol excretion in very low birth weight infants. European Journal of Pediatrics 2016;175(3):365‐72. [PUBMED: 26475348] - PubMed
References to studies awaiting assessment
Calisici 2014 {published data only}
-
- Calisici E, Yarci E, Degirmencioglu H, Oncel MY, Oguz SS, Uras N, et al. PO‐0731 The effects of early oral vitamin a treatment on the prevention of bronchopulmonary dysplasia in the low birth weight infants. Archives of Disease in Childhood 2014;99(Suppl 2):A494. [DOI: 10.1136/archdischild-2014-307384.1371] - DOI
References to ongoing studies
Meyer 2013 {published data only}
-
- Meyer S, Gortner L, Monz D, Maurer E, Nohr D, Biesalski HK, NeoVitaA Study Group. Vitamin A administration using nasogastric tubes. Early Human Development 2014;90(10):625‐6. - PubMed
-
- Meyer S, Gortner L, NeoVitaA Trial Investigators. Early postnatal additional high‐dose oral vitamin A supplementation versus placebo for 28 days for preventing bronchopulmonary dysplasia or death in extremely low birth weight infants. Neonatology 2014;105(3):182‐8. [PUBMED: 24434948] - PubMed
-
- Meyer S, Kronfeld K, Gräber S, Butzer R, Wahl H, Gortner L. Vitamin A to prevent bronchopulmonary dysplasia: the NeoVitaA trial. Journal of Maternal‐fetal & Neonatal Medicine 2013;26(5):544‐5. - PubMed
Additional references
Bates 1995
-
- Bates CJ. Vitamin A. Lancet 1995;345(8941):31‐5. - PubMed
Chabra 1994
-
- Chabra S, Arnold JD, Leslie GI, Bowen JR, Earl J, Wood F. Vitamin A status in preterm neonates with and without chronic lung disease. Journal of Paediatrics and Child Health 1994;30(5):432‐5. - PubMed
Chytil 1992
-
- Chytil F. The lungs and vitamin A. American Journal of Physiology 1992;262(5 Pt 1):L517‐27. - PubMed
Georgieff 1989
-
- Georgieff MK, Mammel MC, Mills MM, Gunter EW, Johnson DE, Thompson TR. Effect of postnatal steroid administration on serum vitamin A concentrations in newborn infants with respiratory compromise. Journal of Pediatrics 1989;114(2):301‐4. - PubMed
GRADEpro‐GDT 2013 [Computer program]
-
- Grading of Recommendations Assessment, Development and Evaluation (GRADE) Working Group. GRADEpro‐GDT. GRADE working group, 2013.
Greene 1987
-
- Greene HL, Phillips BL, Franck L, Fillmore CM, Said HM, Murrell JE, et al. Persistently low retinol levels during and after parenteral feeding of very low birthweight infants; examination of losses into intravenous administration sets and a method of prevention by addition to a lipid emulsion. Pediatrics 1987;79(6):894‐900. - PubMed
Higgins 2011
-
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org,.
Huiming 2005
Humphrey 1996
-
- Humphrey JH, Agoestina T, Wu L, Usman A, Nurachim M, Subardja D, et al. Impact of neonatal vitamin A supplementation on infant morbidity and mortality. Journal of Pediatrics 1996;128(4):489‐96. - PubMed
Hustead 1984
-
- Hustead VA, Gutcher GR, Anderson SA, Zachman RD. Relationship of vitamin A (retinol) status to lung disease in the preterm infant. Journal of Pediatrics 1984;104(4):610‐5. - PubMed
Kennedy 1997
-
- Kennedy KA, Stoll BJ, Ehrenkranz RA, Oh W, Wright LL, Stevenson DK, et al. Vitamin A to prevent bronchopulmonary dysplasia in very‐low‐birth‐weight infants: has the dose been too low? The NICHD Neonatal Research Network. Early Human Development 1997;49(1):19‐31. - PubMed
Lorch 1994
-
- Lorch V, Gaylord MS. Vitamin A and bronchopulmonary dysplasia. Journal of Pediatrics 1994;124(2):328‐9. - PubMed
Mactier 2005
Papile 1978
-
- Papile LA, Burstein J, Burstein R, Koffler H. Incidence and evolution of subependymal and intraventricular hemorrhage: a study of infants with birth weights less than 1,500 gm. Journal of Pediatrics 1978;92(4):529‐34. - PubMed
Review Manager 5 [Computer program]
-
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2008.
Schünemann 2013
-
- Schünemann H, Brożek J, Guyatt G, Oxman A, editors. The GRADE Working Group. GRADE handbook for grading quality of evidence and strength of recommendations [Updated October 2013]. Available from www.guidelinedevelopment.org/handbook.
Shenai 1985
-
- Shenai JP, Chytil F, Stahlman MT. Vitamin A status of neonates with bronchopulmonary dysplasia. Pediatric Research 1985;19(2):185‐8. - PubMed
Shenai 1990
-
- Shenai JP, Rush MG, Stahlman MT, Chytil F. Plasma retinol‐binding protein response to vitamin A administration in infants susceptible to bronchopulmonary dysplasia. Journal of Pediatrics 1990;116(4):607‐14. - PubMed
Shenai 1993
-
- Shenai JP. Vitamin A. In: Tsang RC, Lucas A, Uauy R, Zlotkin S editor(s). Nutritional Needs of the Preterm Infant: Scientific Basis and Practical Guidelines. Baltimore: Williams and Wilkins, 1993:87‐100.
Spears 2004
-
- Spears K, Cheney C, Zerzan J. Low plasma retinol concentrations increase the risk of developing bronchopulmonary dysplasia and long‐term respiratory disability in very‐low‐birth‐weight infants. American Journal of Clinical Nutrition 2004;80(6):1589‐94. - PubMed
Zachman 1996
-
- Zachman RD, Samuels DP, Brand JM, Winston JF, Pi JT. Use of the intramuscular relative‐dose‐response test to predict bronchopulmonary dysplasia in premature infants. American Journal of Clinical Nutrition 1996;63(1):123‐9. - PubMed
References to other published versions of this review
Darlow 1998
Darlow 2000
Darlow 2002
Darlow 2007
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

