Endovenous laser ablation versus mechanochemical ablation with ClariVein(®) in the management of superficial venous insufficiency (LAMA trial): study protocol for a randomised controlled trial
- PMID: 27552990
- PMCID: PMC4995808
- DOI: 10.1186/s13063-016-1548-1
Endovenous laser ablation versus mechanochemical ablation with ClariVein(®) in the management of superficial venous insufficiency (LAMA trial): study protocol for a randomised controlled trial
Abstract
Background: Endovenous thermal techniques, such as endovenous laser ablation (EVLA), are the recommended treatment for truncal varicose veins. However, a disadvantage of thermal techniques is that it requires the administration of tumescent anaesthesia, which can be uncomfortable. Non-thermal, non-tumescent techniques, such as mechanochemical ablation (MOCA) have potential benefits. MOCA combines physical damage to endothelium using a rotating wire, with the infusion of a liquid sclerosant. Preliminary experiences with MOCA showed good results and less post-procedural pain.
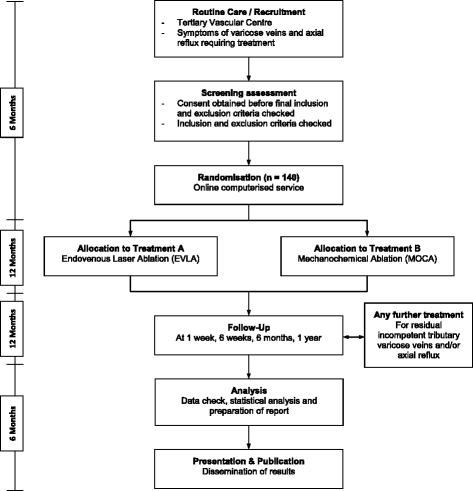
Methods/design: The Laser Ablation versus Mechanochemical Ablation (LAMA) trial is a single-centre randomised controlled trial in which 140 patients will be randomly allocated to EVLA or MOCA. All patients with primary truncal superficial venous insufficiency (SVI) who meet the eligibility criteria will be invited to participate in this trial. The primary outcomes are intra-procedural pain and technical efficacy at 1 year, defined as complete occlusion of target vein segment and assessed using duplex ultrasound. Secondary outcomes are post-procedural pain, analgesia use, procedure time, clinical severity, generic and disease-specific quality of life, bruising, complications, satisfaction, cosmesis, time taken to return to daily activities and/or work, and cost-effectiveness analysis following EVLA or MOCA. Both groups will be evaluated on an intention-to-treat basis.
Discussion: The aim of the LAMA trial is to establish whether MOCA is superior to the current first-line treatment, EVLA. The two main hypotheses are that MOCA may cause less initial pain and disability allowing a more acceptable treatment with an enhanced recovery. The second hypothesis is that this may come at a cost of decreased efficacy, which may lead to increased recurrence and affect longer term quality of life, increasing the requirement for secondary procedures.
Trial registration: ClinicalTrials.gov identifier: NCT02627846 , registered 8 December 2015 EudraCT number: 2015-000730-30 REC ref: 15/YH/0207 R&D ref: R1788.
Keywords: ClariVein®; EVLA; Endovenous laser ablation; MOCA; Mechanochemical ablation; Outcome; Superficial venous insufficiency; Therapy; Treatment; Varicose vein.
Figures
References
-
- Van den Oever R, Hepp B, Debbaut B, Simon I. Socio-economic impact of chronic venous insufficiency. An underestimated public health problem. Int Angiol. 1998;17:161–7. - PubMed
-
- National Institute for Health and Care Excellence. Varicose veins in the legs: the diagnosis and management of varicose veins. Clinical guideline 168. 2013. https://www.nice.org.uk/guidance/cg168. Accessed 28 Apr 2016. - PubMed
-
- Gloviczki P, Comerota AJ, Dalsing MC, Eklof BG, Gillespie DL, Gloviczki ML, Lohr JM, McLafferty RB, Meissner MH, Murad MH, Padberg FT, Pappas PJ, Passman MA, Raffetto JD, Vasquez MA, Wakefield TW, Society for Vascular Surgery, American Venous Forum The care of patients with varicose veins and associated chronic venous diseases: clinical practice guidelines of the Society for Vascular Surgery and the American Venous Forum. J Vasc Surg. 2011;53:2S–48. doi: 10.1016/j.jvs.2011.01.079. - DOI - PubMed
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical