S-Sulfhydration of ATP synthase by hydrogen sulfide stimulates mitochondrial bioenergetics
- PMID: 27553984
- PMCID: PMC5107138
- DOI: 10.1016/j.phrs.2016.08.023
S-Sulfhydration of ATP synthase by hydrogen sulfide stimulates mitochondrial bioenergetics
Abstract
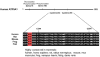
Mammalian cells can utilize hydrogen sulfide (H2S) to support mitochondrial respiration. The aim of our study was to explore the potential role of S-sulfhydration (a H2S-induced posttranslational modification, also known as S-persulfidation) of the mitochondrial inner membrane protein ATP synthase (F1F0 ATP synthase/Complex V) in the regulation of mitochondrial bioenergetics. Using a biotin switch assay, we have detected S-sulfhydration of the α subunit (ATP5A1) of ATP synthase in response to exposure to H2S in vitro. The H2S generator compound NaHS induced S-sulfhydration of ATP5A1 in HepG2 and HEK293 cell lysates in a concentration-dependent manner (50-300μM). The activity of immunocaptured mitochondrial ATP synthase enzyme isolated from HepG2 and HEK293 cells was stimulated by NaHS at low concentrations (10-100nM). Site-directed mutagenesis of ATP5A1 in HEK293 cells demonstrated that cysteine residues at positions 244 and 294 are subject to S-sulfhydration. The double mutant ATP synthase protein (C244S/C294S) showed a significantly reduced enzyme activity compared to control and the single-cysteine-mutated recombinant proteins (C244S or C294S). To determine whether endogenous H2S plays a role in the basal S-sulfhydration of ATP synthase in vivo, we compared liver tissues harvested from wild-type mice and mice deficient in cystathionine-gamma-lyase (CSE, one of the three principal mammalian H2S-producing enzymes). Significantly reduced S-sulfhydration of ATP5A1 was observed in liver homogenates of CSE-/- mice, compared to wild-type mice, suggesting a physiological role for CSE-derived endogenous H2S production in the S-sulfhydration of ATP synthase. Various forms of critical illness (including burn injury) upregulate H2S-producing enzymes and stimulate H2S biosynthesis. In liver tissues collected from mice subjected to burn injury, we detected an increased S-sulfhydration of ATP5A1 at the early time points post-burn. At later time points (when systemic H2S levels decrease) S-sulfhydration of ATP5A1 decreased as well. In conclusion, H2S induces S-sulfhydration of ATP5A1 at C244 and C294. This post-translational modification may be a physiological mechanism to maintain ATP synthase in a physiologically activated state, thereby supporting mitochondrial bioenergetics. The sulfhydration of ATP synthase may be a dynamic process, which may be regulated by endogenous H2S levels under various pathophysiological conditions.
Keywords: ATP synthase; Bioenergetics; Burn; Burn injury; Cysteine; H(2)S; Hydrogen sulfide; Mitochondria; S-Sulfhydration.
Copyright © 2016 Elsevier Ltd. All rights reserved.
Conflict of interest statement
The authors declare no conflicts of interest in relationship to this study.
Figures
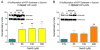
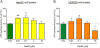





References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

