An ultrasensitive electrogenerated chemiluminescence-based immunoassay for specific detection of Zika virus
- PMID: 27554037
- PMCID: PMC4995374
- DOI: 10.1038/srep32227
An ultrasensitive electrogenerated chemiluminescence-based immunoassay for specific detection of Zika virus
Abstract
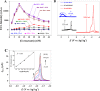
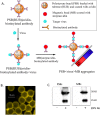
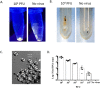
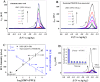
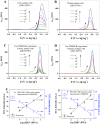
Zika virus (ZIKV) is a globally emerging mosquito-transmitted flavivirus that can cause severe fetal abnormalities, including microcephaly. As such, highly sensitive, specific, and cost-effective diagnostic methods are urgently needed. Here, we report a novel electrogenerated chemiluminescence (ECL)-based immunoassay for ultrasensitive and specific detection of ZIKV in human biological fluids. We loaded polystyrene beads (PSB) with a large number of ECL labels and conjugated them with anti-ZIKV monoclonal antibodies to generate anti-ZIKV-PSBs. These anti-ZIKV-PSBs efficiently captured ZIKV in solution forming ZIKV-anti-ZIKV-PSB complexes, which were subjected to measurement of ECL intensity after further magnetic beads separation. Our results show that the anti-ZIKV-PSBs can capture as little as 1 PFU of ZIKV in 100 μl of saline, human plasma, or human urine. This platform has the potential for development as a cost-effective, rapid and ultrasensitive assay for the detection of ZIKV and possibly other viruses in clinical diagnosis, epidemiologic and vector surveillance, and laboratory research.
Figures
Similar articles
-
A Multiplex Microsphere Immunoassay for Zika Virus Diagnosis.EBioMedicine. 2017 Feb;16:136-140. doi: 10.1016/j.ebiom.2017.01.008. Epub 2017 Jan 10. EBioMedicine. 2017. PMID: 28094237 Free PMC article.
-
Development of a novel peptide aptamer-based immunoassay to detect Zika virus in serum and urine.Theranostics. 2018 Jun 7;8(13):3629-3642. doi: 10.7150/thno.25955. eCollection 2018. Theranostics. 2018. PMID: 30026871 Free PMC article.
-
Detection of Specific ZIKV IgM in Travelers Using a Multiplexed Flavivirus Microsphere Immunoassay.Viruses. 2018 May 12;10(5):253. doi: 10.3390/v10050253. Viruses. 2018. PMID: 29757218 Free PMC article.
-
Zika virus epidemic: an update.Expert Rev Anti Infect Ther. 2016 Dec;14(12):1127-1138. doi: 10.1080/14787210.2016.1245614. Epub 2016 Oct 21. Expert Rev Anti Infect Ther. 2016. PMID: 27712118 Review.
-
How does Zika virus cause microcephaly?Genes Dev. 2017 May 1;31(9):849-861. doi: 10.1101/gad.298216.117. Genes Dev. 2017. PMID: 28566536 Free PMC article. Review.
Cited by
-
An effective live-attenuated Zika vaccine candidate with a modified 5' untranslated region.NPJ Vaccines. 2023 Apr 1;8(1):50. doi: 10.1038/s41541-023-00650-w. NPJ Vaccines. 2023. PMID: 37005424 Free PMC article.
-
A single snapshot multiplex immunoassay platform utilizing dense test lines based on engineered beads.Biosens Bioelectron. 2021 Oct 15;190:113388. doi: 10.1016/j.bios.2021.113388. Epub 2021 May 31. Biosens Bioelectron. 2021. PMID: 34098362 Free PMC article.
-
Highly Sensitive and Selective Direct Detection of Zika Virus Particles in Human Bodily Fluids for Accurate Early Diagnosis of Infection.ACS Omega. 2019 Apr 30;4(4):6808-6818. doi: 10.1021/acsomega.9b00374. Epub 2019 Apr 15. ACS Omega. 2019. PMID: 31058250 Free PMC article.
-
Nanoparticle-enhanced electrical detection of Zika virus on paper microchips.Nanoscale. 2018 Jul 5;10(25):11841-11849. doi: 10.1039/c8nr01646a. Nanoscale. 2018. PMID: 29881853 Free PMC article.
-
Adaptive time modulation technique for multiplexed on-chip particle detection across scales.Optica. 2023 Jul 20;10(7):812-818. doi: 10.1364/optica.489068. Epub 2023 Jun 22. Optica. 2023. PMID: 38818330 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

