An IL-17-dominant immune profile is shared across the major orphan forms of ichthyosis
- PMID: 27554821
- PMCID: PMC8033419
- DOI: 10.1016/j.jaci.2016.07.019
An IL-17-dominant immune profile is shared across the major orphan forms of ichthyosis
Abstract
Background: The ichthyoses are rare genetic disorders associated with generalized scaling, erythema, and epidermal barrier impairment. Pathogenesis-based therapy is largely lacking because the underlying molecular basis is poorly understood.
Objective: We sought to characterize molecularly cutaneous inflammation and its correlation with clinical and barrier characteristics.
Methods: We analyzed biopsy specimens from 21 genotyped patients with ichthyosis (congenital ichthyosiform erythroderma, n = 6; lamellar ichthyosis, n = 7; epidermolytic ichthyosis, n = 5; and Netherton syndrome, n = 3) using immunohistochemistry and RT-PCR and compared them with specimens from healthy control subjects, patients with atopic dermatitis (AD), and patients with psoriasis. Clinical measures included the Ichthyosis Area Severity Index (IASI), which integrates erythema (IASI-E) and scaling (IASI-S); transepidermal water loss; and pruritus.
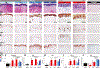
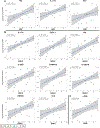
Results: Ichthyosis samples showed increased epidermal hyperplasia (increased thickness and keratin 16 expression) and T-cell and dendritic cell infiltrates. Increases of general inflammatory (IL-2), innate (IL-1β), and some TH1/interferon (IFN-γ) markers in patients with ichthyosis were comparable with those in patients with psoriasis or AD. TNF-α levels in patients with ichthyosis were increased only in those with Netherton syndrome but were much lower than in patients with psoriasis and those with AD. Expression of TH2 cytokines (IL-13 and IL-31) was similar to that seen in control subjects. The striking induction of IL-17-related genes or markers synergistically induced by IL-17 and TNF-α (IL-17A/C, IL-19, CXCL1, PI3, CCL20, and IL36G; P < .05) in patients with ichthyosis was similar to that seen in patients with psoriasis. IASI and IASI-E scores strongly correlated with IL-17A (r = 0.74, P < .001) and IL-17/TNF-synergistic/additive gene expression. These markers also significantly correlated with transepidermal water loss, suggesting a link between the barrier defect and inflammation in patients with ichthyosis.
Conclusion: Our data associate a shared TH17/IL-23 immune fingerprint with the major orphan forms of ichthyosis and raise the possibility of IL-17-targeting strategies.
Keywords: Epidermis; IL-17; Netherton syndrome; TNF-α; autosomal recessive congenital ichthyosis; congenital ichthyosiform erythroderma; epidermolytic ichthyosis; ichthyosis; inflammation; lamellar ichthyosis; skin.
Copyright © 2016 American Academy of Allergy, Asthma & Immunology. Published by Elsevier Inc. All rights reserved.
Figures




References
-
- Oji V, Tadini G, Akiyama M, Blanchet Bardon C, Bodemer C, Bourrat E, et al. Revised nomenclature and classification of inherited ichthyoses: results of the First Ichthyosis Consensus Conference in Soreze 2009. J Am Acad Dermatol 2010; 63:607–41. - PubMed
-
- DiGiovanna JJ, Robinson-Bostom L. Ichthyosis: etiology, diagnosis, and management. Am J Clin Dermatol 2003; 4:81–95. - PubMed
-
- Oji V, Traupe H. Ichthyoses: differential diagnosis and molecular genetics. Eur J Dermatol 2006; 16:349–59. - PubMed
-
- Craig WY, Roberson M, Palomaki GE, Shackleton CH, Marcos J, Haddow JE. Prevalence of steroid sulfatase deficiency in California according to race and ethnicity. Prenat Diagn 2010; 30:893–8. - PubMed
-
- Traupe H, Fischer J, Oji V. Nonsyndromic types of ichthyoses - an update. J Dtsch Dermatol Ges 2014; 12:109–21. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

