The inhibitory mechanism of Cordyceps sinensis on cigarette smoke extract-induced senescence in human bronchial epithelial cells
- PMID: 27555762
- PMCID: PMC4968689
- DOI: 10.2147/COPD.S107396
The inhibitory mechanism of Cordyceps sinensis on cigarette smoke extract-induced senescence in human bronchial epithelial cells
Abstract
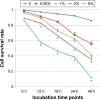
Objectives: Cellular senescence is a state of irreversible growth arrest induced either by telomere shortening (replicative senescence) or stress. The bronchial epithelial cell is often injured by inhaled toxic substances, such as cigarette smoke. In the present study, we investigated whether exposure to cigarette smoke extract (CSE) induces senescence of bronchial epithelial cells; and Cordyceps sinensis mechanism of inhibition of CSE-induced cellular senescence.
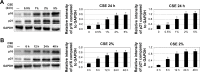
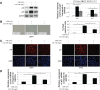
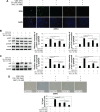
Methods: Human bronchial epithelial cells (16HBE cells) cultured in vitro were treated with CSE and/or C. sinensis. p16, p21, and senescence-associated-galactosidase activity were used to detect cellular senescence with immunofluorescence, quantitative polymerase chain reaction, and Western blotting. Reactive oxygen species (ROS), PI3K/AKT/mTOR and their phosphorylated proteins were examined to testify the activation of signaling pathway by ROS fluorescent staining and Western blotting. Then, inhibitors of ROS and PI3K were used to further confirm the function of this pathway.
Results: Cellular senescence was upregulated by CSE treatment, and C. sinensis can decrease CSE-induced cellular senescence. Activation of ROS/PI3K/AKT/mTOR signaling pathway was enhanced by CSE treatment, and decreased when C. sinensis was added. Blocking ROS/PI3K/AKT/mTOR signaling pathway can attenuate CSE-induced cellular senescence.
Conclusion: CSE can induce cellular senescence in human bronchial epithelial cells, and ROS/PI3K/AKT/mTOR signaling pathway may play an important role in this process. C. sinensis can inhibit the CSE-induced senescence.
Keywords: COPD; Cordyceps sinensis; ROS/PI3K/AKT/mTOR signaling pathway; senescence.
Figures



Similar articles
-
Mitochondrial fragmentation in cigarette smoke-induced bronchial epithelial cell senescence.Am J Physiol Lung Cell Mol Physiol. 2013 Nov 15;305(10):L737-46. doi: 10.1152/ajplung.00146.2013. Epub 2013 Sep 20. Am J Physiol Lung Cell Mol Physiol. 2013. PMID: 24056969
-
Beta-elemene alleviates cigarette smoke-triggered inflammation, apoptosis, and oxidative stress in human bronchial epithelial cells, and refrains the PI3K/AKT/mTOR signaling pathway.Allergol Immunopathol (Madr). 2024 Nov 1;52(6):79-84. doi: 10.15586/aei.v52i6.1199. eCollection 2024. Allergol Immunopathol (Madr). 2024. PMID: 39515800
-
Involvement of creatine kinase B in cigarette smoke-induced bronchial epithelial cell senescence.Am J Respir Cell Mol Biol. 2012 Mar;46(3):306-12. doi: 10.1165/rcmb.2011-0214OC. Epub 2011 Oct 6. Am J Respir Cell Mol Biol. 2012. PMID: 21980054
-
Cellular and Molecular Signatures of Oxidative Stress in Bronchial Epithelial Cell Models Injured by Cigarette Smoke Extract.Int J Mol Sci. 2022 Feb 4;23(3):1770. doi: 10.3390/ijms23031770. Int J Mol Sci. 2022. PMID: 35163691 Free PMC article. Review.
-
Autophagy in the Cellular Consequences of Tobacco Smoking: Insights into Senescence.J Biochem Mol Toxicol. 2024 Dec;38(12):e70065. doi: 10.1002/jbt.70065. J Biochem Mol Toxicol. 2024. PMID: 39588771 Review.
Cited by
-
Scutellaria baicalensis and Their Natural Flavone Compounds as Potential Medicinal Drugs for the Treatment of Nicotine-Induced Non-Small-Cell Lung Cancer and Asthma.Int J Environ Res Public Health. 2021 May 14;18(10):5243. doi: 10.3390/ijerph18105243. Int J Environ Res Public Health. 2021. PMID: 34069141 Free PMC article. Review.
-
Behavioral and Cognitive Performance Following Exposure to Second-Hand Smoke (SHS) from Tobacco Products Associated with Oxidative-Stress-Induced DNA Damage and Repair and Disruption of the Gut Microbiome.Genes (Basel). 2023 Aug 27;14(9):1702. doi: 10.3390/genes14091702. Genes (Basel). 2023. PMID: 37761842 Free PMC article.
-
Targeting p16-induced senescence prevents cigarette smoke-induced emphysema by promoting IGF1/Akt1 signaling in mice.Commun Biol. 2019 Aug 9;2:307. doi: 10.1038/s42003-019-0532-1. eCollection 2019. Commun Biol. 2019. PMID: 31428695 Free PMC article.
-
Nucleosides isolated from Ophiocordyceps sinensis inhibit cigarette smoke extract-induced inflammation via the SIRT1-nuclear factor-κB/p65 pathway in RAW264.7 macrophages and in COPD mice.Int J Chron Obstruct Pulmon Dis. 2018 Sep 10;13:2821-2832. doi: 10.2147/COPD.S172579. eCollection 2018. Int J Chron Obstruct Pulmon Dis. 2018. PMID: 30237706 Free PMC article.
-
The mechanism of action of Ophiocordyceps sinensis mycelia for prevention of acute lung injury based on non-targeted serum metabolomics.PLoS One. 2023 Jun 16;18(6):e0287331. doi: 10.1371/journal.pone.0287331. eCollection 2023. PLoS One. 2023. PMID: 37327224 Free PMC article.
References
-
- Global Initiative for Chronic Obstructive Lung Disease (GOLD) Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease. [Accessed April 16, 2014]. Updated 2014. Available from: http://www.goldcopd.org/uploads/users/files/GOLD_Report_2014.pdf. - PubMed
-
- Kumar M, Seeger W, Voswinckel R. Senescence-associated secretory phenotype and its possible role in chronic obstructive pulmonary disease. Am J Respir Cell Mol Biol. 2014;51(3):323–333. - PubMed
-
- Manuel C, Blasco MA, Manuel S. Cellular senescence in cancer and aging. Cell. 2007;130(2):223–233. - PubMed
-
- Barnes PJ. Mechanisms of development of multimorbidity in the elderly. Eur Respir J. 2015;45(3):790–806. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

