RANK and RANK ligand expression in primary human osteosarcoma
- PMID: 27556008
- PMCID: PMC4986823
- DOI: 10.1016/j.jbo.2015.06.002
RANK and RANK ligand expression in primary human osteosarcoma
Abstract
Receptor activator of nuclear factor kappa-B ligand (RANKL) is an essential mediator of osteoclast formation, function and survival. In patients with solid tumor metastasis to the bone, targeting the bone microenvironment by inhibition of RANKL using denosumab, a fully human monoclonal antibody (mAb) specific to RANKL, has been demonstrated to prevent tumor-induced osteolysis and subsequent skeletal complications. Recently, a prominent functional role for the RANKL pathway has emerged in the primary bone tumor giant cell tumor of bone (GCTB). Expression of both RANKL and RANK is extremely high in GCTB tumors and denosumab treatment was associated with tumor regression and reduced tumor-associated bone lysis in GCTB patients. In order to address the potential role of the RANKL pathway in another primary bone tumor, this study assessed human RANKL and RANK expression in human primary osteosarcoma (OS) using specific mAbs, validated and optimized for immunohistochemistry (IHC) or flow cytometry. Our results demonstrate RANKL expression was observed in the tumor element in 68% of human OS using IHC. However, the staining intensity was relatively low and only 37% (29/79) of samples exhibited≥10% RANKL positive tumor cells. RANK expression was not observed in OS tumor cells. In contrast, RANK expression was clearly observed in other cells within OS samples, including the myeloid osteoclast precursor compartment, osteoclasts and in giant osteoclast cells. The intensity and frequency of RANKL and RANK staining in OS samples were substantially less than that observed in GCTB samples. The observation that RANKL is expressed in OS cells themselves suggests that these tumors may mediate an osteoclastic response, and anti-RANKL therapy may potentially be protective against bone pathologies in OS. However, the absence of RANK expression in primary human OS cells suggests that any autocrine RANKL/RANK signaling in human OS tumor cells is not operative, and anti-RANKL therapy would not directly affect the tumor.
Keywords: APC, allophycocyanin; ATCC, American Type Culture Collection; Antibodies; ELISA, enzyme linked immunosorbent assay; FACS, fluorescence-activated cell sorting; FBS, fetal bovine serum; FFPE, formalin-fixed, paraffin-embedded; GCTB, giant cell tumor of bone; Human osteosarcoma; IHC, immunohistochemistry; ISH, in situ hybridization; IgG1, immunoglobulin G1; Immunohistochemistry; LN, lymph node; OS, osteosarcoma; Protein expression; RANK; RANK, receptor activator of nuclear factor kappa-B; RANKL; RANKL, receptor activator of nuclear factor kappa-B ligand; RNA, ribonucleic acid; RT-PCR, reverse transcriptase polymerase chain reaction; cDNA, complementary deoxyribonucleic acid; mAb, monoclonal antibody; mRNA, messenger ribonucleic acid.
Figures


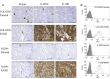




References
-
- Klein M.J., Siegal G.P. Osteosarcoma: anatomic and histologic variants. Am J Clin Pathol. 2006;125:555–581. - PubMed
-
- Mutsaers A.J., Walkley C.R. Cells of origin in osteosarcoma: mesenchymal stem cells or osteoblast committed cells? Bone. 2014;62:56–63. - PubMed
-
- Botter S.M., Neri D., Fuchs B. Recent advances in osteosarcoma. Curr Opin Pharmacol. 2014;16:15–23. - PubMed
-
- Lacey D.L., Boyle W.J., Simonet W.S., Kostenuik P.J., Dougall W.C., Sullivan J.K. Bench to bedside: elucidation of the OPG-RANK-RANKL pathway and the development of denosumab. Nat Rev Drug Discov. 2012;11:401–419. - PubMed
-
- Stopeck A.T., Lipton A., Body J.J., Steger G.G., Tonkin K., de Boer R.H. Denosumab compared with zoledronic acid for the treatment of bone metastases in patients with advanced breast cancer: a randomized, double-blind study. J Clin Oncol. 2010;28:5132–5139. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

