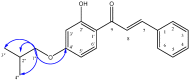
Synthesis and Evaluation of Novel Oxyalkylated Derivatives of 2',4'-Dihydroxychalcone as Anti-Oomycete Agents against Bronopol Resistant Strains of Saprolegnia sp
- PMID: 27556457
- PMCID: PMC5000761
- DOI: 10.3390/ijms17081366
Synthesis and Evaluation of Novel Oxyalkylated Derivatives of 2',4'-Dihydroxychalcone as Anti-Oomycete Agents against Bronopol Resistant Strains of Saprolegnia sp
Abstract
A series of novel oxyalkylchalcones substituted with alkyl groups were designed and synthesized, and the antioomycete activity of the series was evaluated in vitro against Saprolegnia strains. All tested O-alkylchalcones were synthesized by means of nucleophilic substitution from the natural compound 2',4'-dihydroxychalcone (1) and the respective alkyl bromide. The natural chalcone (1) and 10 synthetic oxyalkylchalcones (2-11) were tested against Saprolegnia parasitica and Saprolegnia australis. Among synthetic analogs, 2-hydroxy,4-farnesyloxychalcone (11) showed the most potent activity against Saprolegnia sp., with MIC and MOC values of 125 µg/mL (similar to bronopol at 150 µg/mL) and 175 µg/mL, respectively; however, 2',4'-dihydroxychalcone (1) was the strongest and most active molecule, with MIC and MOC values of 6.25 µg/mL and 12.5 µg/mL.
Keywords: Saprolegnia australis; Saprolegnia parasitica; fish pathogen; oxyalkylchalcones.
Figures
References
-
- Bruyère C., Genovese S., Lallemand B., Ionescu-Motatu A., Curini M., Kiss R., Epifano F. Growth inhibitory activities of oxyprenylated and non-prenylated naturally occurring phenylpropanoids in cancer cell lines. Bioorg. Med. Chem. Lett. 2011;21:4174–4179. doi: 10.1016/j.bmcl.2011.05.089. - DOI - PubMed
-
- Prusky D., Keen N.T. Involvement of preformed antifungal compounds in the resistance of subtropical fruits to fungal decay. Plant Dis. 1993;77:114–119. doi: 10.1094/PD-77-0114. - DOI
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources