Modification of Pulsed Electric Field Conditions Results in Distinct Activation Profiles of Platelet-Rich Plasma
- PMID: 27556645
- PMCID: PMC4996457
- DOI: 10.1371/journal.pone.0160933
Modification of Pulsed Electric Field Conditions Results in Distinct Activation Profiles of Platelet-Rich Plasma
Abstract
Background: Activated autologous platelet-rich plasma (PRP) used in therapeutic wound healing applications is poorly characterized and standardized. Using pulsed electric fields (PEF) to activate platelets may reduce variability and eliminate complications associated with the use of bovine thrombin. We previously reported that exposing PRP to sub-microsecond duration, high electric field (SMHEF) pulses generates a greater number of platelet-derived microparticles, increased expression of prothrombotic platelet surfaces, and differential release of growth factors compared to thrombin. Moreover, the platelet releasate produced by SMHEF pulses induced greater cell proliferation than plasma.
Aims: To determine whether sub-microsecond duration, low electric field (SMLEF) bipolar pulses results in differential activation of PRP compared to SMHEF, with respect to profiles of activation markers, growth factor release, and cell proliferation capacity.
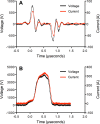
Methods: PRP activation by SMLEF bipolar pulses was compared to SMHEF pulses and bovine thrombin. PRP was prepared using the Harvest SmartPreP2 System from acid citrate dextrose anticoagulated healthy donor blood. PEF activation by either SMHEF or SMLEF pulses was performed using a standard electroporation cuvette preloaded with CaCl2 and a prototype instrument designed to take into account the electrical properties of PRP. Flow cytometry was used to assess platelet surface P-selectin expression, and annexin V binding. Platelet-derived growth factor (PDGF), vascular endothelial growth factor (VEGF), endothelial growth factor (EGF) and platelet factor 4 (PF4), and were measured by ELISA. The ability of supernatants to stimulate proliferation of human epithelial cells in culture was also evaluated. Controls included vehicle-treated, unactivated PRP and PRP with 10 mM CaCl2 activated with 1 U/mL bovine thrombin.
Results: PRP activated with SMLEF bipolar pulses or thrombin had similar light scatter profiles, consistent with the presence of platelet-derived microparticles, platelets, and platelet aggregates whereas SMHEF pulses primarily resulted in platelet-derived microparticles. Microparticles and platelets in PRP activated with SMLEF bipolar pulses had significantly lower annexin V-positivity than those following SMHEF activation. In contrast, the % P-selectin positivity and surface P-selectin expression (MFI) for platelets and microparticles in SMLEF bipolar pulse activated PRP was significantly higher than that in SMHEF-activated PRP, but not significantly different from that produced by thrombin activation. Higher levels of EGF were observed following either SMLEF bipolar pulses or SMHEF pulses of PRP than after bovine thrombin activation while VEGF, PDGF, and PF4 levels were similar with all three activating conditions. Cell proliferation was significantly increased by releasates of both SMLEF bipolar pulse and SMHEF pulse activated PRP compared to plasma alone.
Conclusions: PEF activation of PRP at bipolar low vs. monopolar high field strength results in differential platelet-derived microparticle production and activation of platelet surface procoagulant markers while inducing similar release of growth factors and similar capacity to induce cell proliferation. Stimulation of PRP with SMLEF bipolar pulses is gentler than SMHEF pulses, resulting in less platelet microparticle generation but with overall activation levels similar to that obtained with thrombin. These results suggest that PEF provides the means to alter, in a controlled fashion, PRP properties thereby enabling evaluation of their effects on wound healing and clinical outcomes.
Conflict of interest statement
AST, AC, CAM and VBN are employees of GE Healthcare. ALG is a former employee of GE Healthcare. ALF received research support from GE Healthcare. The remaining authors declare no competing interests. This does not alter the authors’ adherence to PLOS ONE policies on sharing data and materials.
Figures


Similar articles
-
Platelet-rich plasma stimulated by pulse electric fields: Platelet activation, procoagulant markers, growth factor release and cell proliferation.Platelets. 2016;27(2):128-35. doi: 10.3109/09537104.2015.1048214. Epub 2015 Jun 1. Platelets. 2016. PMID: 26030682
-
Tunable activation of therapeutic platelet-rich plasma by pulse electric field: Differential effects on clot formation, growth factor release, and platelet morphology.PLoS One. 2018 Sep 26;13(9):e0203557. doi: 10.1371/journal.pone.0203557. eCollection 2018. PLoS One. 2018. PMID: 30256831 Free PMC article.
-
Use of a Cyclooxygenase-2 Inhibitor Does Not Inhibit Platelet Activation or Growth Factor Release From Platelet-Rich Plasma.Am J Sports Med. 2017 Dec;45(14):3351-3357. doi: 10.1177/0363546517730578. Epub 2017 Sep 27. Am J Sports Med. 2017. PMID: 28952781
-
Platelet-rich preparations to improve healing. Part II: platelet activation and enrichment, leukocyte inclusion, and other selection criteria.J Oral Implantol. 2014 Aug;40(4):511-21. doi: 10.1563/AAID-JOI-D-12-00106. J Oral Implantol. 2014. PMID: 25106017 Review.
-
Platelet-Rich Plasma in Tissue Engineering: Hype and Hope.Eur Surg Res. 2018;59(3-4):265-275. doi: 10.1159/000492415. Epub 2018 Sep 21. Eur Surg Res. 2018. PMID: 30244245 Review.
Cited by
-
[Research progress of metal micro-battery dressings in wound repair].Zhonghua Shao Shang Yu Chuang Mian Xiu Fu Za Zhi. 2023 Jun 20;39(6):596-600. doi: 10.3760/cma.j.cn501225-20220926-00416. Zhonghua Shao Shang Yu Chuang Mian Xiu Fu Za Zhi. 2023. PMID: 37805778 Free PMC article. Review. Chinese.
-
Sustained Release of Transforming Growth Factor-β1 from Platelet-Rich Chondroitin Sulfate Glycosaminoglycan Gels.J Knee Surg. 2018 May;31(5):410-415. doi: 10.1055/s-0037-1603801. Epub 2017 Jun 23. J Knee Surg. 2018. PMID: 28645130 Free PMC article.
-
Design, characterization and experimental validation of a compact, flexible pulsed power architecture for ex vivo platelet activation.PLoS One. 2017 Jul 26;12(7):e0181214. doi: 10.1371/journal.pone.0181214. eCollection 2017. PLoS One. 2017. PMID: 28746392 Free PMC article.
-
Autologous platelet-rich plasma for healing chronic venous leg ulcers: Clinical efficacy and potential mechanisms.Int Wound J. 2019 Jun;16(3):788-792. doi: 10.1111/iwj.13098. Epub 2019 Mar 12. Int Wound J. 2019. PMID: 30864220 Free PMC article.
-
Mechanisms of electrical vasoconstriction.J Neuroeng Rehabil. 2018 May 29;15(1):43. doi: 10.1186/s12984-018-0390-y. J Neuroeng Rehabil. 2018. PMID: 29843762 Free PMC article.
References
-
- Klement GL, Shai S, Varon D (2013) The role of platelets in angiogenesis In: Michelson AD, editor. Platelets. 3rd ed. San Diego: Elsevier/Academic Press; pp. 487–502.
-
- Driver VR, Hanft J, Fylling CP, Beriou JM, Autologel Diabetic Foot Ulcer Study Group (2006) A prospective, randomized, controlled trial of autologous platelet-rich plasma gel for the treatment of diabetic foot ulcers. Ostomy Wound Management 52: 68–74. - PubMed
-
- Bielecki TM, Gazdzik TS, Arendt J, Szczepanski T, Krol W, Wielkoszynski T (2007) Antibacterial effect of autologous platelet gel enriched with growth factors and other active substances: an in vitro study. J Bone Joint Surg Br 89: 417–420. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

