Amnioinfusion for chorioamnionitis
- PMID: 27556818
- PMCID: PMC6507500
- DOI: 10.1002/14651858.CD011622.pub2
Amnioinfusion for chorioamnionitis
Abstract
Background: Chorioamnionitis is a leading cause of perinatal morbidity and mortality. Amnioinfusion aims at reducing the adverse effects of chorioamnionitis by dilution of the infective organisms or by an anti-microbial effect of the fluid infused.
Objectives: The objective of this review was to determine the effect of amnioinfusion on clinical and sub-clinical chorioamnionitis, fetal well-being, fetal heart rate characteristics and perinatal and maternal morbidity and mortality.
Search methods: We searched the Cochrane Pregnancy and Childbirth Group's Trials Register (6 July 2016), PubMed, ClinicalTrials.gov, the WHO International Clinical Trials Registry Platform (ICTRP) (6 July 2016) and reference lists of retrieved studies.
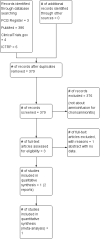
Selection criteria: Randomised clinical trials (RCTs) of amnioinfusion (treatment group) versus no amnioinfusion in women with chorioamnionitis.We would have also considered trials comparing amnioinfusion with sham amnioinfusion; different types or volumes of amnioinfusion fluid but none were identified.Cluster-RCTs and quasi-RCTs were eligible for inclusion but none were identified. We identified one study published in abstract form but it did not contain any numerical data and has therefore been excluded. Studies using a cross-over design are not an appropriate study design and thus were not eligible for inclusion in this review.
Data collection and analysis: Two review authors independently assessed potential studies for inclusion and assessed trial quality. Both review authors independently extracted data and data were checked for accuracy.
Main results: We included one small trial (with data from 34 participants) comparing transcervical amnioinfusion with no amnioinfusion. The trial was considered to be at a high risk of bias overall, due to small numbers, inconsistency in the reporting and lack of information on blinding. Meta-analysis was not possible. Transcervical amnioinfusion was with room temperature saline at 10 mL per minute for 60 minutes, then 3 mL per minute until delivery versus no amnioinfusion. All women received intrauterine pressure catheter, acetaminophen and antibiotics (ampicillin or, if receiving Group B beta streptococcal prophylaxis, penicillin and gentamycin). We did not identify any trials that used transabdominal amnioinfusion.Compared to no amnioinfusion, transcervical amnioinfusion had no clear effect on the incidence of postpartum endometritis (risk ratio (RR) 1.50, 95% confidence interval (CI) 0.29 to 7.87; absolute risk 176/1000 (95% CI 34 to 96) versus 118/1000;low-quality evidence). Nor was there a clear effect in the incidence of neonatal infection (RR 3.00, 95% CI 0.13 to 68.84; absolute risk 0/1000 (95% CI 0 to 0) versus 0/1000; low-quality evidence). The outcome of perinatal death or severe morbidity (such as neonatal encephalopathy, intraventricular haemorrhage, admission to intensive/high care) was not reported in the included trial.In terms of this review's secondary outcomes, the rate of caesarean section was the same in both groups (RR 1.00, 95% CI 0.35 to 2.83; absolute risk 294/1000 (95% CI 103 to 832) versus 294/1000; low-quality evidence). There was no clear difference in the duration of maternal antibiotic treatment between the amnioinfusion and no amnioinfusion control group (mean difference (MD) 16 hours, 95% CI -1.75 to 33.75); nor in the duration of hospitalisation (MD 3.00 hours, 95% CI -15.49 to 21.49). The study did not report any information about how many babies had a low Apgar score at five minutes after birth.Women in the amnioinfusion group had a lower temperature at delivery compared to women in the control group (MD -0.38°C, 95% CI -0.74 to -0.02) but this outcome was not pre-specified in the protocol for this review.The majority of this review's secondary outcomes were not reported in the included study.
Authors' conclusions: There is insufficient evidence to fully evaluate the effectiveness of using transcervical amnioinfusion for chorioamnionitis and to assess the safety of this intervention or women's satisfaction. We did not identify any trials that used transabdominal amnioinfusion. The evidence in this review can neither support nor refute the use of transcervical amnioinfusion outside of clinical trials. We included one small study that reported on a limited number of outcomes of interest in this review. The numbers included in this review are too small for meaningful assessment of substantive outcomes, where reported. For those outcomes we assessed using GRADE (postpartum endometritis, neonatal infection, and caesarean section), we downgraded the quality of the evidence to low - with downgrading decisions based on small numbers and a lack of information on blinding. The included study did not report on this review's other primary outcome (perinatal death or severe morbidity).The reduction in pyrexia, though not a pre-specified outcome of this review, may be of relevance in terms of benefits to the fetus of reduced exposure to heat. We postulate that the temperature reduction found may be a direct cooling effect of amnioinfusion with room temperature fluid, rather than reduction of infection. Larger trials are needed to confirm and extend the findings of the trial reviewed here. These should be randomised controlled trials; participants, women with chorioamnionitis; interventions, amnioinfusion; comparisons, no amnioinfusion; outcomes, maternal and perinatal outcomes including neurodevelopmental measures.Further research is justified to determine possible benefits or risks of amnioinfusion for chorioamnionitis, and to investigate possible benefits of reducing temperature in fetuses considered at risk of neurological damage. Research should include randomised trials to examine transcervical or transabdominal amnioinfusion compared with no infusion for chorioamnionitis and examine outcomes listed in the methods of this review.
Conflict of interest statement
G Justus Hofmeyr ‐ receives royalties from UpToDate for chapters related to
Joseph AK Kiiza: none known.
Figures






Update of
References
References to studies included in this review
Parilla 1998 {published data only}
-
- McDermott TM, Parilla BV. Amnioinfusion as a therapy to reduce post partum endometriosis after chorioamnionitis. American Journal of Obstetrics and Gynecology 1998;178(1 Pt 2):S212.
-
- Parilla BV, McDermott TM. Prophylactic amnioinfusion in pregnancies complicated by chorioamnionitis: a prospective randomized trial. American Journal of Perinatology 1998;15(12):649‐52. - PubMed
References to studies excluded from this review
Macri 1993 {published data only}
-
- Macri C, Paek J, Greenspoon J, Paul R. Antibiotic amnioinfusion does not decrease maternal morbidity in term pregnancy complicated by amnionitis. American Journal of Obstetrics and Gynecology 1993;168:414.
Additional references
Asakura 2004
-
- Asakura H. Fetal and neonatal thermoregulation. Journal of Nippon Medical School 2004;71(6):360‐70. - PubMed
DiGiulio 2012
-
- DiGiulio DB. Diversity of microbes in amniotic fluid. Seminars in Fetal & Neonatal Medicine 2012;17(1):2‐11. - PubMed
Fishman 2012
-
- Fishman SG, Gelber SE. Evidence for the clinical management of chorioamnionitis. Seminars in Fetal & Neonatal Medicine 2012;17(1):46‐50. - PubMed
Gericke G1989
-
- Gericke GS, Hofmeyr GJ, Laburn H, Isaacs H. Does heat damage fetuses?. Medical Hypotheses 1989;29(4):275‐8. - PubMed
Gibbs 1982
-
- Gibbs RS, Blanco JD, Clair PJ, Castaneda YS. Quantitative bacteriology of amniotic fluid from women with clinical intraamniotic infection at term. Journal of Infectious Diseases 1982;145(1):1‐8. - PubMed
Gibbs 1988
-
- Gibbs RS, Dinsmoor MJ, Newyon ER, Ramamurthy RS. A randomised trial of intrapartum versus immediate postpartum treatment of women with intra‐amniotic infection. Obstetrics & Gynecology 1988;72(6):823‐8. - PubMed
Gilstrap 1988
-
- Gilstrap LC 3rd, Leveno KJ, Cox SM, Burris JS, Mashburn M, Rosenfeld CR. Intrapartum treatment of acute chorioamnionitis: impact on neonatal sepsis. American Journal of Obstetrics and Gynecology 1988;159(3):579‐83. - PubMed
Higgins 2011
-
- Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Hofmeyr 2012
Hofmeyr 2014
Hofmeyr 2014b
Kozinszky 2014
-
- Kozinszky Z, Sikovanyecz J, Pásztor N. Severe midtrimester oligohydramnios: treatment strategies. Current Opinion in Obstetrics & Gynecology 2014;26(2):67‐76. - PubMed
Lau 2005
-
- Lau J, Magee F, Qiu Z, Houbé J, Dadelszen P, Lee SK. Chorioamnionitis with a fetal inflammatory response is associated with higher neonatal mortality, morbidity, and resource use than chorioamnionitis displaying a maternal inflammatory response only. American Journal of Obstetrics and Gynecology 2005;193(3 Pt 1):708‐13. - PubMed
Major 1993
-
- Major CA, Veciana M, Morgan MA, Henry JA. The impact of amnioinfusion on maternal and neonatal morbidity in pregnancies complicated by pre‐term premature rupture of membranes and amnionitis. American Journal of Obstetrics and Gynecology 1993;168(1 Pt 2):SPO Abstract no: 383.
McDermott 1998
-
- McDermott TM, Parilla BV. Amnioinfusion as a therapy to reduce post partum endometriosis after chorioamnionitis. American Journal of Obstetrics and Gynecology 1998;178(1 Pt 2):S212.
Monohan 1995
-
- Monahan E, Katz V, Cox R. Amnioinfusion for preventing puerperal infection. Journal of Reproductive Medicine 1995;40(10):721‐3. - PubMed
Moore 2008
-
- Moore TM, Callaway CW, Hostler D. Core temperature cooling in healthy volunteers after rapid intravenous infusion of cold and room temperature saline solution. Annals of Emergency Medicine 2008;51(2):153‐9. - PubMed
Newton 1989
-
- Newton ER, Prihoda TJ, Gibbs RS. Logistic regression analysis of risk factors for intra‐amniotic infection. Obstetrics & Gynecology 1989;73(4):571‐5. - PubMed
Owen 1990
-
- Owen J, Henson BV, Hauth JC. A prospective randomized study of saline solution amnioinfusion. American Journal of Obstetrics and Gynecology 1990;162(5):1146‐9. - PubMed
Parilla 1998b
-
- Parilla BV, McDermott TM. Prophylactic amnioinfusion in pregnancies complicated by chorioamnionitis: a prospective randomized trial. American Journal of Perinatology 1998;15(12):649‐52. - PubMed
RevMan 2014 [Computer program]
-
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Roberts 2014
-
- Roberts D, Vause S, Martin W, Green P, Walkinshaw S, Bricker L, et al. Amnioinfusion in preterm premature rupture of membranes (AMIPROM): a randomised controlled trial of amnioinfusion versus expectant management in very early preterm premature rupture of membranes ‐ a pilot study. Health Technology Assessment 2014;18(21):1‐136. - PMC - PubMed
Sciscione 2001
-
- Sciscione AC, Zainia T, Leet T, Winn JN, Winn HN. A new device for measuring intrauterine temperature. American Journal of Obstetrics and Gynecology 2001;184(7):1431‐4. - PubMed
Shah 2007
-
- Shah PS, Ohlsson A, Perlman M. Hypothermia to treat neonatal hypoxic ischemic encephalopathy: systematic review. Archives of Pediatrics & Adolescent Medicine 2007;161(10):951‐8. - PubMed
Tchirikov 2015
-
- Tchirikov M, Zhumadilov Z, Winarno AS, Haase R, Buchmann J. Treatment of preterm premature rupture of membranes with oligo‐/anhydramnion colonized by multiresistant bacteria with continuous amnioinfusion and antibiotic administrations through a subcutaneously implanted intrauterine port system: a case report. Fetal Diagnosis and Therapy 2015;epub:epub. - PubMed
Tomlinson 2012
-
- Tomlinson TM, Schaecher C, Sadovsky Y, Gross G. Intrauterine temperature during intrapartum amnioinfusion: a prospective observational study. BJOG: an international journal of obstetrics and gynaecology 2012;119(8):958‐63. - PubMed
van Teeffelen 2014
Wu 2003
-
- Wu YW, Escobar GJ, Grether JK, Croen LA, Greene JD, Newman TB. Chorioamnionitis and cerebral palsy in term and near‐term infants. JAMA 2003;290(20):2677‐84. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

