HER2 expression identifies dynamic functional states within circulating breast cancer cells
- PMID: 27556950
- PMCID: PMC5161614
- DOI: 10.1038/nature19328
HER2 expression identifies dynamic functional states within circulating breast cancer cells
Abstract
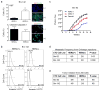
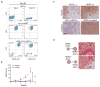
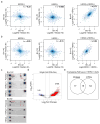
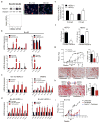
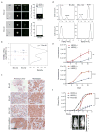
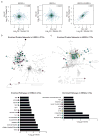
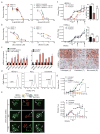
Circulating tumour cells in women with advanced oestrogen-receptor (ER)-positive/human epidermal growth factor receptor 2 (HER2)-negative breast cancer acquire a HER2-positive subpopulation after multiple courses of therapy. In contrast to HER2-amplified primary breast cancer, which is highly sensitive to HER2-targeted therapy, the clinical significance of acquired HER2 heterogeneity during the evolution of metastatic breast cancer is unknown. Here we analyse circulating tumour cells from 19 women with ER+/HER2- primary tumours, 84% of whom had acquired circulating tumour cells expressing HER2. Cultured circulating tumour cells maintain discrete HER2+ and HER2- subpopulations: HER2+ circulating tumour cells are more proliferative but not addicted to HER2, consistent with activation of multiple signalling pathways; HER2- circulating tumour cells show activation of Notch and DNA damage pathways, exhibiting resistance to cytotoxic chemotherapy, but sensitivity to Notch inhibition. HER2+ and HER2- circulating tumour cells interconvert spontaneously, with cells of one phenotype producing daughters of the opposite within four cell doublings. Although HER2+ and HER2- circulating tumour cells have comparable tumour initiating potential, differential proliferation favours the HER2+ state, while oxidative stress or cytotoxic chemotherapy enhances transition to the HER2- phenotype. Simultaneous treatment with paclitaxel and Notch inhibitors achieves sustained suppression of tumorigenesis in orthotopic circulating tumour cell-derived tumour models. Together, these results point to distinct yet interconverting phenotypes within patient-derived circulating tumour cells, contributing to progression of breast cancer and acquisition of drug resistance.
Conflict of interest statement
The authors declare no competing financial interests.
Figures



References
-
- Houssami N, Macaskill P, Balleine RL, Bilous M, Pegram MD. HER2 discordance between primary breast cancer and its paired metastasis: tumor biology or test artefact? Insights through meta-analysis. Breast Cancer Res Treat. 2011;129:659–674. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

