Convergent evolution of defensin sequence, structure and function
- PMID: 27557668
- PMCID: PMC11107677
- DOI: 10.1007/s00018-016-2344-5
Convergent evolution of defensin sequence, structure and function
Abstract
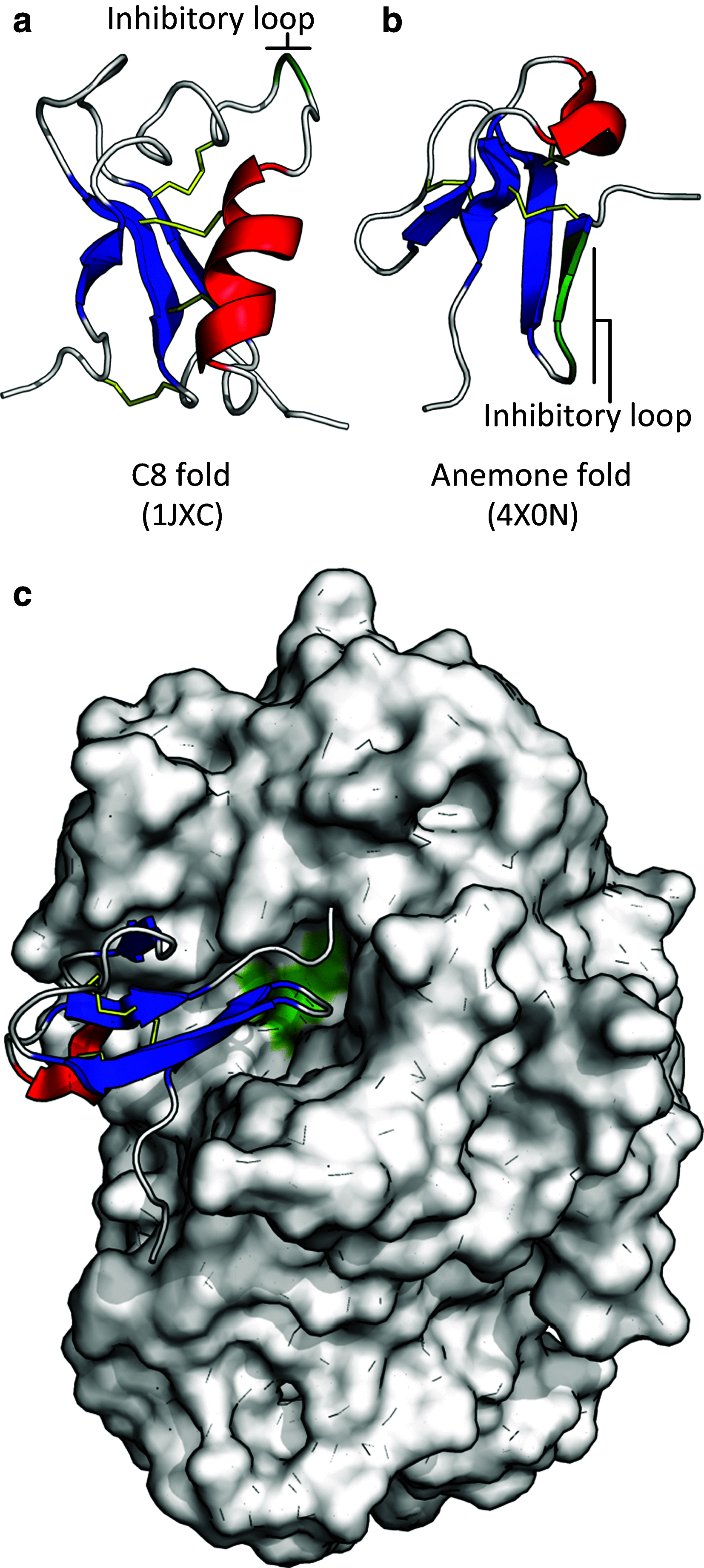
Defensins are a well-characterised group of small, disulphide-rich, cationic peptides that are produced by essentially all eukaryotes and are highly diverse in their sequences and structures. Most display broad range antimicrobial activity at low micromolar concentrations, whereas others have other diverse roles, including cell signalling (e.g. immune cell recruitment, self/non-self-recognition), ion channel perturbation, toxic functions, and enzyme inhibition. The defensins consist of two superfamilies, each derived from an independent evolutionary origin, which have subsequently undergone extensive divergent evolution in their sequence, structure and function. Referred to as the cis- and trans-defensin superfamilies, they are classified based on their secondary structure orientation, cysteine motifs and disulphide bond connectivities, tertiary structure similarities and precursor gene sequence. The utility of displaying loops on a stable, compact, disulphide-rich core has been exploited by evolution on multiple occasions. The defensin superfamilies represent a case where the ensuing convergent evolution of sequence, structure and function has been particularly extreme. Here, we discuss the extent, causes and significance of these convergent features, drawing examples from across the eukaryotes.
Keywords: Antimicrobial peptide; Disulphide-rich protein; Divergent evolution; Evolutionary constraint; Evolvability; Protein superfamily.
Figures
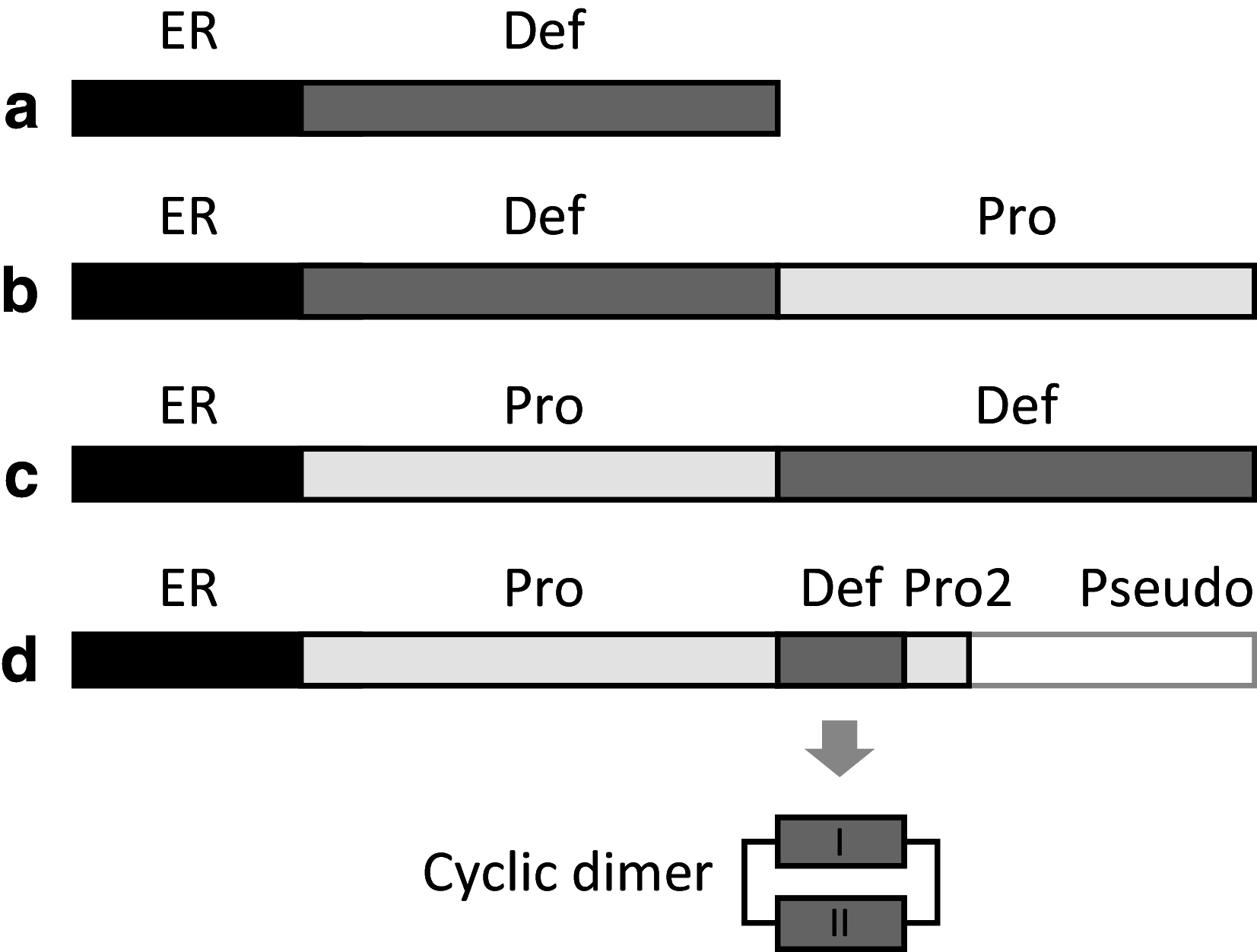



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

