High quality standards for a large-scale prospective population-based observational cohort: Constances
- PMID: 27557750
- PMCID: PMC4997774
- DOI: 10.1186/s12889-016-3439-5
High quality standards for a large-scale prospective population-based observational cohort: Constances
Abstract
Background: Long-term multicentre studies are subject to numerous factors that may affect the integrity of their conclusions. Quality control and standardization of data collection are crucial to minimise the biases induced by these factors. Nevertheless, tools implemented to manage biases are rarely described in publications about population-based cohorts. This report aims to describe the processes implemented to control biases in the Constances cohort taking lung function results as an example.
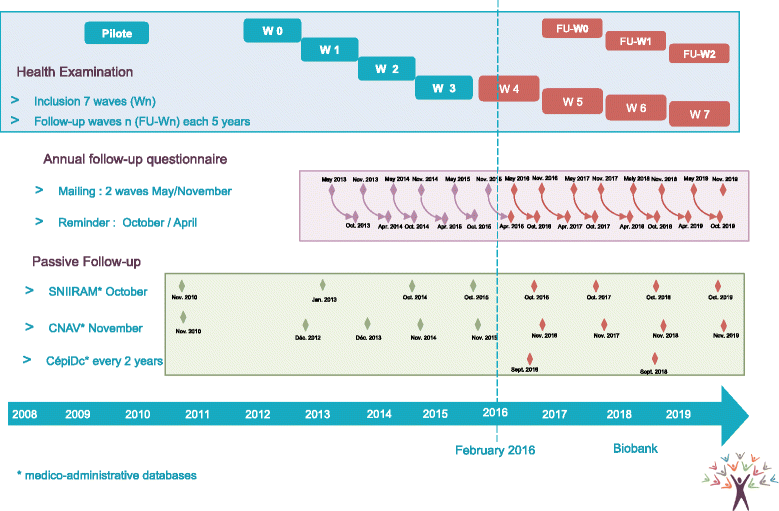
Methods: Constances is a general-purpose population-based cohort of 200,000 participants. Volunteers attend physical examinations at baseline and then every 5 years at selected study sites. Medical device specifications and measurement methods have to comply with Standard Operating Procedures developed by experts. Protocol deviations are assessed by on-site inspections and database controls. In February 2016, more than 94,000 participants yielding around 30 million readings from physical exams, had been covered by our quality program.
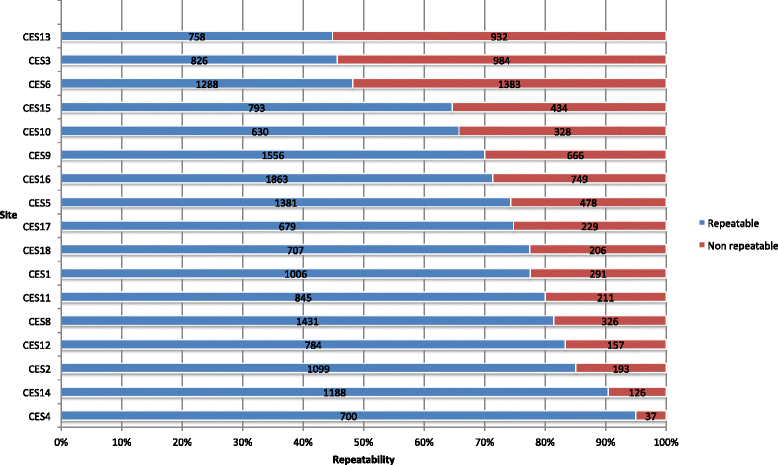
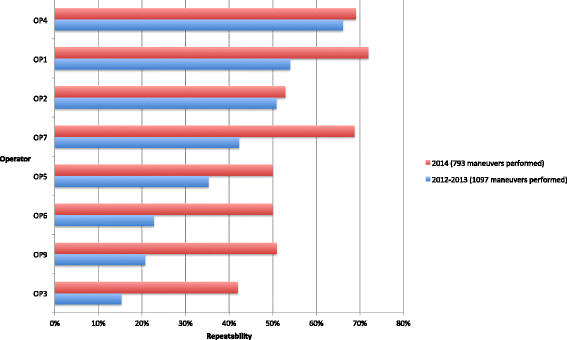
Results: Participating centres accepted to revise their practices in accordance with the study research specifications. Distributors of medical devices were asked to comply with international guidelines and Constances requirements. Close monitoring enhanced the quality of measurements and recordings of the physical exams. Regarding lung function testing, spirometry acceptability rates per operator doubled in some sites within a few months and global repeatability reached 96.7 % for 29,772 acceptable maneuvers.
Conclusions: Despite Constances volunteers being followed in multiple sites with heterogeneous materials, the investment of significant resources to set up and maintain a continuous quality management process has proved effective in preventing drifts and improving accuracy of collected data.
Keywords: Cohort study; Epidemiological methods; Measurement tool development; Methodology; Respiratory.
Figures
References
-
- CNAMTS. [http://www.ameli.fr/. Accessed 14 Mar 2016].
-
- Good Epidemiological Practice (GEP) IEA guidelines for proper conduct of epidemiological research. [http://ieaweb.org/good-epidemiological-practice-gep/. Accessed on 14 Mar 2016].
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

