Impact of genetic modulation of SULT1A enzymes on DNA adduct formation by aristolochic acids and 3-nitrobenzanthrone
- PMID: 27557898
- PMCID: PMC5364269
- DOI: 10.1007/s00204-016-1808-6
Impact of genetic modulation of SULT1A enzymes on DNA adduct formation by aristolochic acids and 3-nitrobenzanthrone
Abstract
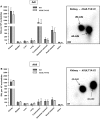
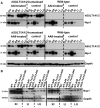
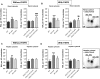
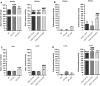
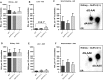
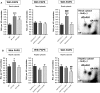
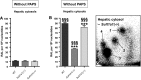
Exposure to aristolochic acid (AA) causes aristolochic acid nephropathy (AAN) and Balkan endemic nephropathy (BEN). Conflicting results have been found for the role of human sulfotransferase 1A1 (SULT1A1) contributing to the metabolic activation of aristolochic acid I (AAI) in vitro. We evaluated the role of human SULT1A1 in AA bioactivation in vivo after treatment of transgenic mice carrying a functional human SULT1A1-SULT1A2 gene cluster (i.e. hSULT1A1/2 mice) and Sult1a1(-/-) mice with AAI and aristolochic acid II (AAII). Both compounds formed characteristic DNA adducts in the intact mouse and in cytosolic incubations in vitro. However, we did not find differences in AAI-/AAII-DNA adduct levels between hSULT1A1/2 and wild-type (WT) mice in all tissues analysed including kidney and liver despite strong enhancement of sulfotransferase activity in both kidney and liver of hSULT1A1/2 mice relative to WT, kidney and liver being major organs involved in AA metabolism. In contrast, DNA adduct formation was strongly increased in hSULT1A1/2 mice compared to WT after treatment with 3-nitrobenzanthrone (3-NBA), another carcinogenic aromatic nitro compound where human SULT1A1/2 is known to contribute to genotoxicity. We found no differences in AAI-/AAII-DNA adduct formation in Sult1a1(-/-) and WT mice in vivo. Using renal and hepatic cytosolic fractions of hSULT1A1/2, Sult1a1(-/-) and WT mice, we investigated AAI-DNA adduct formation in vitro but failed to find a contribution of human SULT1A1/2 or murine Sult1a1 to AAI bioactivation. Our results indicate that sulfo-conjugation catalysed by human SULT1A1 does not play a role in the activation pathways of AAI and AAII in vivo, but is important in 3-NBA bioactivation.
Keywords: 3-Nitrobenzanthrone; Aristolochic acid nephropathy; Balkan endemic nephropathy; Carcinogen metabolism; DNA adducts; Sulfotransferase 1A1.
Figures



References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

