Potency testing of mesenchymal stromal cell growth expanded in human platelet lysate from different human tissues
- PMID: 27557940
- PMCID: PMC4997686
- DOI: 10.1186/s13287-016-0383-3
Potency testing of mesenchymal stromal cell growth expanded in human platelet lysate from different human tissues
Abstract
Background: Mesenchymal stromal cells (MSCs) have been largely investigated, in the past decade, as potential therapeutic strategies for various acute and chronic pathological conditions. MSCs isolated from different sources, such as bone marrow (BM), umbilical cord tissue (UCT) and adipose tissue (AT), share many biological features, although they may show some differences on cumulative yield, proliferative ability and differentiation potential. The standardization of MSCs growth and their functional amplification is a mandatory objective of cell therapies. The aim of this study was to evaluate the cumulative yield and the ex vivo amplification potential of MSCs obtained from various sources and different subjects, using defined culture conditions with a standardized platelet lysate (PL) as growth stimulus.
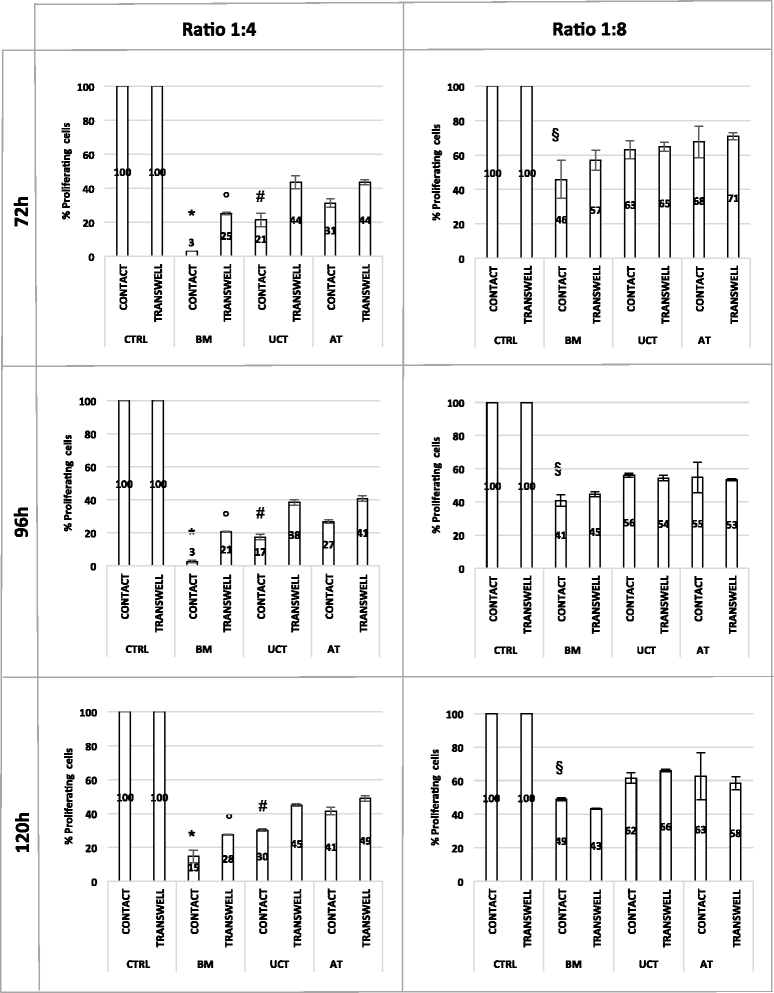
Methods: MSCs isolated from BM, UCT and AT and expanded in human PL were compared in terms of cumulative yield and growth potential per gram of starting tissue. MSCs morphology, phenotype, differentiation potential, and immunomodulatory properties were also investigated to evaluate their biological characteristics.
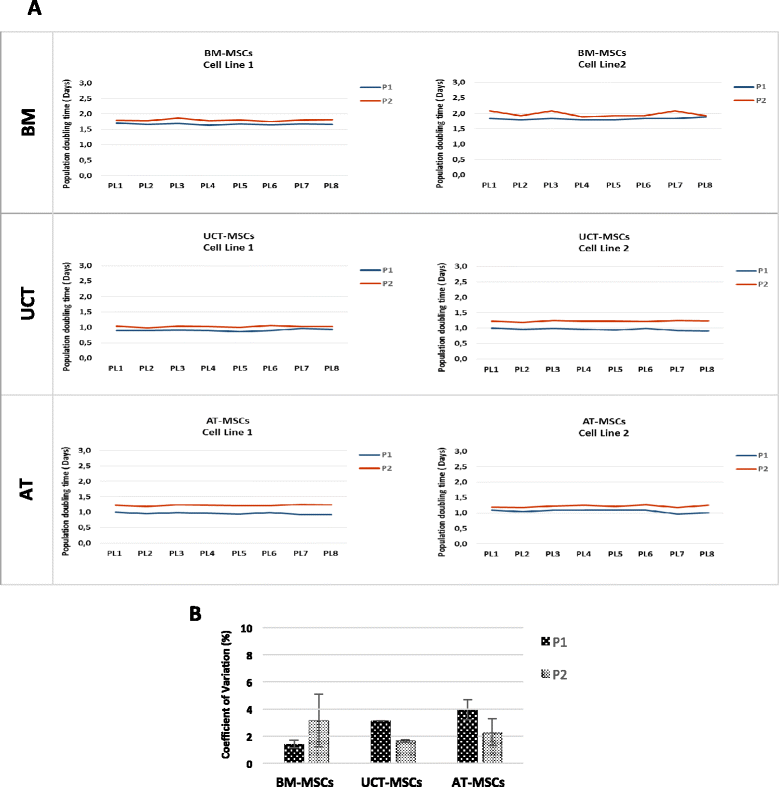
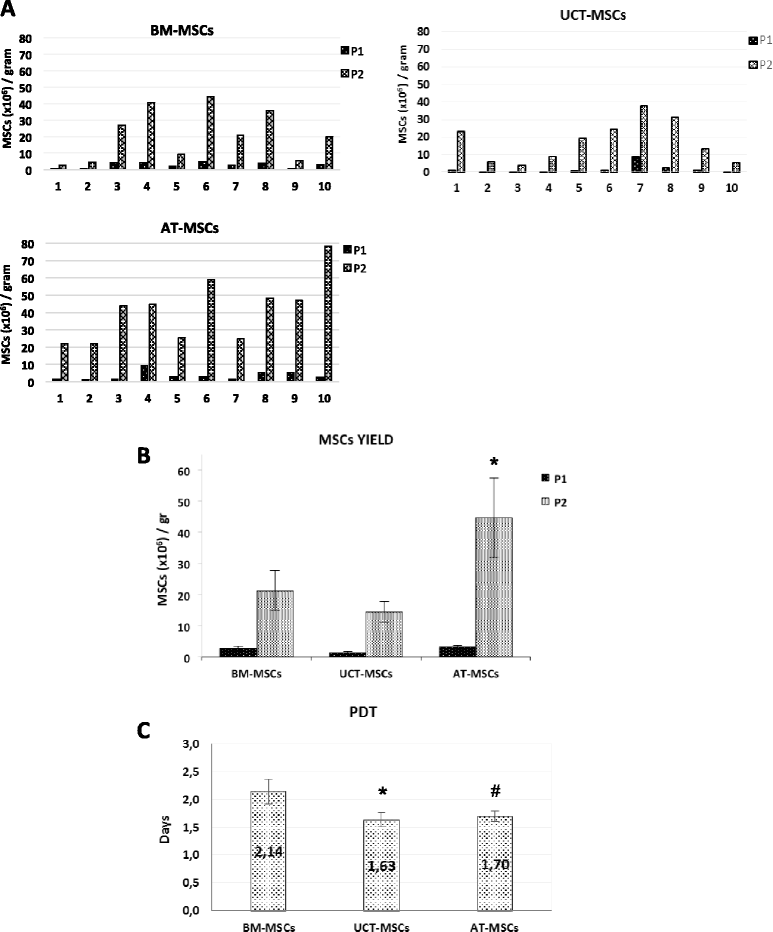
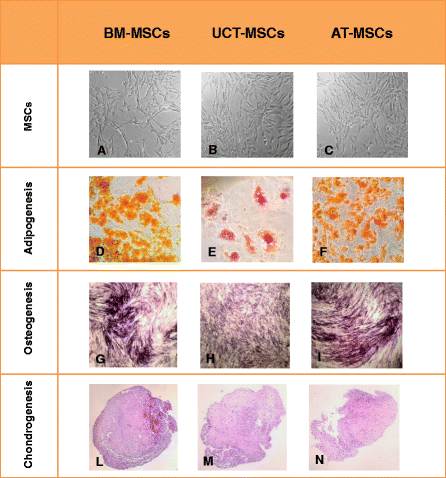
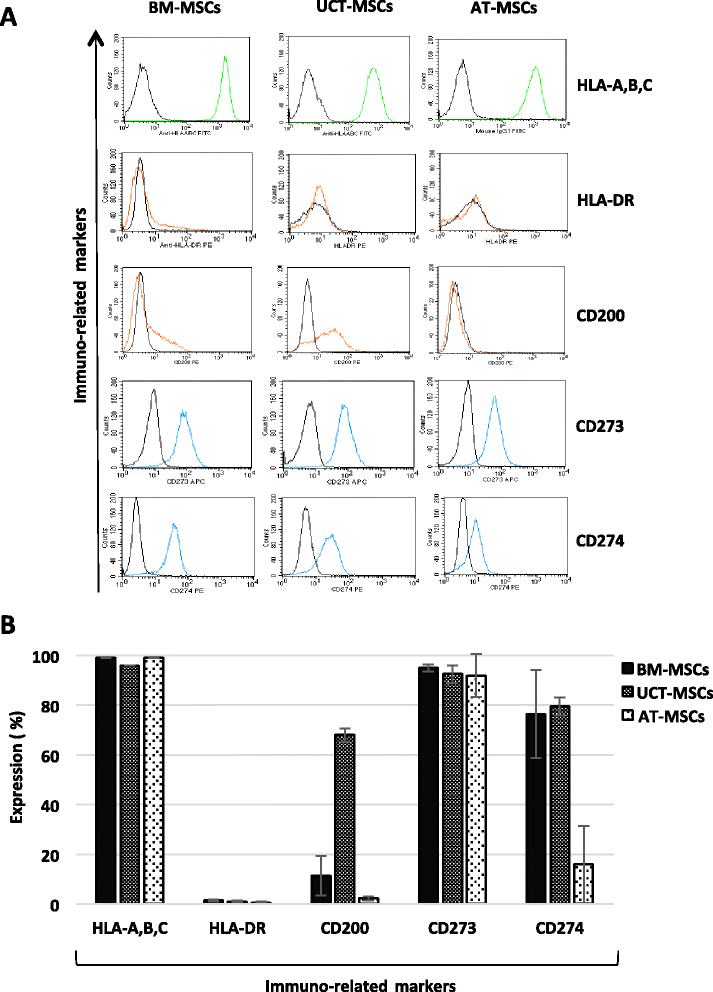
Results: The use of standardized PL-based culture conditions resulted in a very low variability of MSC growth. Our data showed that AT has the greater capacity to generate MSC per gram of initial tissue, compared to BM and UCT. However, UCT-MSCs replicated faster than AT-MSCs and BM-MSCs, revealing a greater proliferation capacity of this source irrespective of its lower MSC yield. All MSCs exhibited the typical MSC phenotype and the ability to differentiate into all mesodermal lineages, while BM-MSCs showed the most prominent immunosuppressive effect in vitro.
Conclusions: The adoption of standardized culture conditions may help researchers and clinicians to reveal particular characteristics and inter-individual variability of MSCs sourced from different tissues. These data will be beneficial to set the standards for tissue collection and MSCs clinical-scale expansion both for cell banking and for cell-based therapy settings.
Keywords: Adipose tissue; Bone marrow; Culture standardization; Mesenchymal stromal cells; Platelet lysate; Proliferative potential; Umbilical cord tissue.
Figures


References
-
- Secunda R, Vennila R, Mohanashankar AM, Rajasundari M, Jeswanth S, Surendran R. Isolation, expansion and characterisation of mesenchymal stem cells from human bone marrow, adipose tissue, umbilical cord blood and matrix: a comparative study. Cytotechnology. 2015;67:793–807. doi: 10.1007/s10616-014-9718-z. - DOI - PMC - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

