Malignant hyperthermia-associated mutations in the S2-S3 cytoplasmic loop of type 1 ryanodine receptor calcium channel impair calcium-dependent inactivation
- PMID: 27558158
- PMCID: PMC5130589
- DOI: 10.1152/ajpcell.00134.2016
Malignant hyperthermia-associated mutations in the S2-S3 cytoplasmic loop of type 1 ryanodine receptor calcium channel impair calcium-dependent inactivation
Abstract
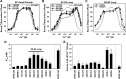
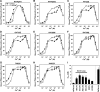
Channel activities of skeletal muscle ryanodine receptor (RyR1) are activated by micromolar Ca2+ and inactivated by higher (∼1 mM) Ca2+ To gain insight into a mechanism underlying Ca2+-dependent inactivation of RyR1 and its relationship with skeletal muscle diseases, we constructed nine recombinant RyR1 mutants carrying malignant hyperthermia or centronuclear myopathy-associated mutations and determined RyR1 channel activities by [3H]ryanodine binding assay. These mutations are localized in or near the RyR1 domains which are responsible for Ca2+-dependent inactivation of RyR1. Four RyR1 mutations (F4732D, G4733E, R4736W, and R4736Q) in the cytoplasmic loop between the S2 and S3 transmembrane segments (S2-S3 loop) greatly reduced Ca2+-dependent channel inactivation. Activities of these mutant channels were suppressed at 10-100 μM Ca2+, and the suppressions were relieved by 1 mM Mg2+ The Ca2+- and Mg2+-dependent regulation of S2-S3 loop RyR1 mutants are similar to those of the cardiac isoform of RyR (RyR2) rather than wild-type RyR1. Two mutations (T4825I and H4832Y) in the S4-S5 cytoplasmic loop increased Ca2+ affinities for channel activation and decreased Ca2+ affinities for inactivation, but impairment of Ca2+-dependent inactivation was not as prominent as those of S2-S3 loop mutants. Three mutations (T4082M, S4113L, and N4120Y) in the EF-hand domain showed essentially the same Ca2+-dependent channel regulation as that of wild-type RyR1. The results suggest that nine RyR1 mutants associated with skeletal muscle diseases were differently regulated by Ca2+ and Mg2+ Four malignant hyperthermia-associated RyR1 mutations in the S2-S3 loop conferred RyR2-type Ca2+- and Mg2+-dependent channel regulation.
Keywords: Ca2+-dependent inactivation; central core disease; centronuclear myopathy; malignant hyperthermia; type-1 ryanodine receptor.
Copyright © 2016 the American Physiological Society.
Figures




Similar articles
-
Structural and functional interactions between the EF hand domain and S2-S3 loop in the type-1 ryanodine receptor ion channel.J Biol Chem. 2024 Feb;300(2):105606. doi: 10.1016/j.jbc.2023.105606. Epub 2023 Dec 28. J Biol Chem. 2024. PMID: 38159862 Free PMC article.
-
Mutations to Gly2370, Gly2373 or Gly2375 in malignant hyperthermia domain 2 decrease caffeine and cresol sensitivity of the rabbit skeletal-muscle Ca2+-release channel (ryanodine receptor isoform 1).Biochem J. 2001 Nov 15;360(Pt 1):97-105. doi: 10.1042/0264-6021:3600097. Biochem J. 2001. PMID: 11695996 Free PMC article.
-
Reduced threshold for store overload-induced Ca2+ release is a common defect of RyR1 mutations associated with malignant hyperthermia and central core disease.Biochem J. 2017 Aug 7;474(16):2749-2761. doi: 10.1042/BCJ20170282. Biochem J. 2017. PMID: 28687594 Free PMC article.
-
Ryanodine receptor mutations in malignant hyperthermia and central core disease.Hum Mutat. 2000;15(5):410-7. doi: 10.1002/(SICI)1098-1004(200005)15:5<410::AID-HUMU2>3.0.CO;2-D. Hum Mutat. 2000. PMID: 10790202 Review.
-
Congenital muscle disorders with cores: the ryanodine receptor calcium channel paradigm.Curr Opin Pharmacol. 2008 Jun;8(3):319-26. doi: 10.1016/j.coph.2008.01.005. Epub 2008 Mar 4. Curr Opin Pharmacol. 2008. PMID: 18313359 Review.
Cited by
-
Structure-based mechanism of RyR channel operation by calcium and magnesium ions.PLoS Comput Biol. 2025 Apr 29;21(4):e1012950. doi: 10.1371/journal.pcbi.1012950. eCollection 2025 Apr. PLoS Comput Biol. 2025. PMID: 40300027 Free PMC article.
-
Three-dimensional perspective on ryanodine receptor mutations causing skeletal and cardiac muscle-related diseases.Curr Opin Pharmacol. 2023 Feb;68:102327. doi: 10.1016/j.coph.2022.102327. Epub 2022 Dec 12. Curr Opin Pharmacol. 2023. PMID: 36516687 Free PMC article. Review.
-
Ryanodine Receptor Structure and Function in Health and Disease.Subcell Biochem. 2018;87:329-352. doi: 10.1007/978-981-10-7757-9_11. Subcell Biochem. 2018. PMID: 29464565 Free PMC article. Review.
-
Interplay between Mg2+ and Ca2+ at multiple sites of the ryanodine receptor.Nat Commun. 2024 May 15;15(1):4115. doi: 10.1038/s41467-024-48292-3. Nat Commun. 2024. PMID: 38750013 Free PMC article.
-
Fitness Costs of Chlorantraniliprole Resistance Related to the SeNPF Overexpression in the Spodoptera exigua (Lepidoptera: Noctuidae).Int J Mol Sci. 2021 May 10;22(9):5027. doi: 10.3390/ijms22095027. Int J Mol Sci. 2021. PMID: 34068540 Free PMC article.
References
-
- Anderson AA, Brown RL, Polster B, Pollock N, Stowell KM. Identification and biochemical characterization of a novel ryanodine receptor gene mutation associated with malignant hyperthermia. Anesthesiology 108: 208–215, 2008. - PubMed
-
- Brown RL, Pollock AN, Couchman KG, Hodges M, Hutchinson DO, Waaka R, Lynch P, McCarthy TV, Stowell KM. A novel ryanodine receptor mutation and genotype-phenotype correlation in a large malignant hyperthermia New Zealand Maori pedigree. Hum Mol Genet 9: 1515–1524, 2000. - PubMed
-
- Chugun A, Sato O, Takeshima H, Ogawa Y. Mg2+ activates the ryanodine receptor type 2 (RyR2) at intermediate Ca2+ concentrations. Am J Physiol Cell Physiol 292: C535–C544, 2007. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

