Autophagy-associated dengue vesicles promote viral transmission avoiding antibody neutralization
- PMID: 27558165
- PMCID: PMC4997566
- DOI: 10.1038/srep32243
Autophagy-associated dengue vesicles promote viral transmission avoiding antibody neutralization
Abstract
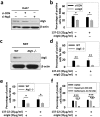
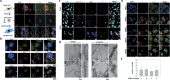
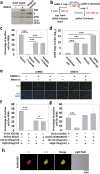
One of the major defense mechanisms against virus spread in vivo is the blocking of viral infectibility by neutralizing antibodies. We describe here the identification of infectious autophagy-associated dengue vesicles released from infected cells. These vesicles contain viral proteins E, NS1, prM/M, and viral RNA, as well as host lipid droplets and LC3-II, an autophagy marker. The viral RNA can be protected within the autophagic organelles since anti-dengue neutralizing antibodies do not have an effect on the vesicle-mediated transmission that is able to initiate a new round of infection in target cells. Importantly, such infectious vesicles were also detected in a patient serum. Our study suggests that autophagy machinery plays a new role in dengue virus transmission. This discovery explains the inefficiency of neutralizing antibody upon dengue infection as a potential immune evasion mechanism in vivo.
Figures


References
-
- Guzman M. G. & Harris E. Dengue. Lancet 385, 453–465 (2015). - PubMed
-
- Screaton G., Mongkolsapaya J., Yacoub S. & Roberts C. New insights into the immunopathology and control of dengue virus infection. Nat. Rev. Immunol. 15, 745–759 (2015). - PubMed
-
- Simmons C. P. et al.. Recent advances in dengue pathogenesis and clinical management. Vaccine 33, 7061–7068 (2015). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

