Activation of microglia by retroviral infection correlates with transient clearance of prions from the brain but does not change incubation time
- PMID: 27558169
- PMCID: PMC6625757
- DOI: 10.1111/bpa.12441
Activation of microglia by retroviral infection correlates with transient clearance of prions from the brain but does not change incubation time
Abstract
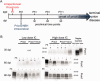
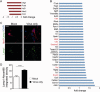
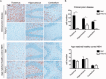
Prion diseases are fatal transmissible diseases, where conversion of the endogenous prion protein (PrPC ) into a misfolded isoform (PrPSc ) leads to neurodegeneration. Microglia, the immune cells of the brain, are activated in neurodegenerative disorders including prion diseases; however, their impact on prion disease pathophysiology is unclear with both beneficial PrPSc -clearing and detrimental potentially neurotoxic effects. Moreover, monocytes entering the brain from the periphery during disease course might add to disease pathophysiology. Here, the degree of microglia activation in the brain of prion infected mice with and without an additional intraperitoneal retrovirus infection was studied. Peripheral murine retrovirus infection leads to activation of parenchymal microglia without recruitment of monocytes. This activation correlated with transient clearance or delay in accumulation of infectious prions specifically from the brain at early time points in the diseases course. Microglia expression profiling showed upregulation of genes involved in protein degradation coinciding with prion clearance. This enforces a concept where microglia act beneficial in prion disease if adequately activated. Once microglia activation has ceased, prion disease reemerges leading to disease kinetics undistinguishable from the situation in prion-only infected mice. This might be caused by the loss of microglial homeostatic function at clinical prion disease.
Keywords: clearance; microglia; microglia signature; monocytes; neurodegeneration; prion disease; prions; protein misfolding; retrovirus.
© 2016 International Society of Neuropathology.
Figures



Similar articles
-
Inflammatory response of microglia to prions is controlled by sialylation of PrPSc.Sci Rep. 2018 Jul 27;8(1):11326. doi: 10.1038/s41598-018-29720-z. Sci Rep. 2018. PMID: 30054538 Free PMC article.
-
Persistent retroviral infection with MoMuLV influences neuropathological signature and phenotype of prion disease.Acta Neuropathol. 2012 Jul;124(1):111-26. doi: 10.1007/s00401-012-0944-1. Epub 2012 Jan 24. Acta Neuropathol. 2012. PMID: 22271154
-
Neuroinflammation, Microglia, and Cell-Association during Prion Disease.Viruses. 2019 Jan 15;11(1):65. doi: 10.3390/v11010065. Viruses. 2019. PMID: 30650564 Free PMC article. Review.
-
Flow Cytometric Detection of PrPSc in Neurons and Glial Cells from Prion-Infected Mouse Brains.J Virol. 2017 Dec 14;92(1):e01457-17. doi: 10.1128/JVI.01457-17. Print 2018 Jan 1. J Virol. 2017. PMID: 29046463 Free PMC article.
-
Loss of Homeostatic Microglia Signature in Prion Diseases.Cells. 2022 Sep 21;11(19):2948. doi: 10.3390/cells11192948. Cells. 2022. PMID: 36230910 Free PMC article. Review.
Cited by
-
The Effects of Immune System Modulation on Prion Disease Susceptibility and Pathogenesis.Int J Mol Sci. 2020 Oct 2;21(19):7299. doi: 10.3390/ijms21197299. Int J Mol Sci. 2020. PMID: 33023255 Free PMC article. Review.
-
Absence of Apolipoprotein E is associated with exacerbation of prion pathology and promotes microglial neurodegenerative phenotype.Acta Neuropathol Commun. 2021 Sep 26;9(1):157. doi: 10.1186/s40478-021-01261-z. Acta Neuropathol Commun. 2021. PMID: 34565486 Free PMC article.
-
MicroRNA-30e-5p Regulates SOCS1 and SOCS3 During Bacterial Infection.Front Cell Infect Microbiol. 2021 Jan 27;10:604016. doi: 10.3389/fcimb.2020.604016. eCollection 2020. Front Cell Infect Microbiol. 2021. PMID: 33585275 Free PMC article.
-
Inflammatory response of microglia to prions is controlled by sialylation of PrPSc.Sci Rep. 2018 Jul 27;8(1):11326. doi: 10.1038/s41598-018-29720-z. Sci Rep. 2018. PMID: 30054538 Free PMC article.
-
Complement 3+-astrocytes are highly abundant in prion diseases, but their abolishment led to an accelerated disease course and early dysregulation of microglia.Acta Neuropathol Commun. 2019 May 22;7(1):83. doi: 10.1186/s40478-019-0735-1. Acta Neuropathol Commun. 2019. PMID: 31118110 Free PMC article.
References
-
- Aguzzi A, Rajendran L (2009) The transcellular spread of cytosolic amyloids, prions, and prionoids. Neuron 64:783–790. - PubMed
-
- Alais S, Simoes S, Baas D, Lehmann S, Raposo G, Darlix JL, Leblanc P (2008) Mouse neuroblastoma cells release prion infectivity associated with exosomal vesicles. Biol Cell 100:603–615. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

