Metal-responsive promoter DNA compaction by the ferric uptake regulator
- PMID: 27558202
- PMCID: PMC5007355
- DOI: 10.1038/ncomms12593
Metal-responsive promoter DNA compaction by the ferric uptake regulator
Abstract
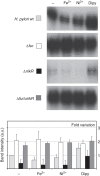
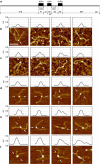
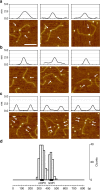
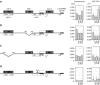
Short-range DNA looping has been proposed to affect promoter activity in many bacterial species and operator configurations, but only few examples have been experimentally investigated in molecular detail. Here we present evidence for a metal-responsive DNA condensation mechanism controlled by the Helicobacter pylori ferric uptake regulator (Fur), an orthologue of the widespread Fur family of prokaryotic metal-dependent regulators. H. pylori Fur represses the transcription of the essential arsRS acid acclimation operon through iron-responsive oligomerization and DNA compaction, encasing the arsR transcriptional start site in a repressive macromolecular complex. A second metal-dependent regulator NikR functions as nickel-dependent anti-repressor at this promoter, antagonizing the binding of Fur to the operator elements responsible for the DNA condensation. The results allow unifying H. pylori metal ion homeostasis and acid acclimation in a mechanistically coherent model, and demonstrate, for the first time, the existence of a selective metal-responsive DNA compaction mechanism controlling bacterial transcriptional regulation.
Figures



References
-
- Dorman C. J. Genome architecture and global gene regulation in bacteria: making progress towards a unified model? Nat. Rev. Microbiol. 11, 349–355 (2013). - PubMed
-
- Schleif R. DNA looping. Annu. Rev. Biochem. 61, 199–223 (1992). - PubMed
-
- Hochschild A., Douhan J. & Ptashne M. How lambda repressor and lambda Cro distinguish between OR1 and OR3. Cell 47, 807–816 (1986). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

