Neutralizing Antibody Responses to Antigenically Drifted Influenza A(H3N2) Viruses among Children and Adolescents following 2014-2015 Inactivated and Live Attenuated Influenza Vaccination
- PMID: 27558294
- PMCID: PMC5051070
- DOI: 10.1128/CVI.00297-16
Neutralizing Antibody Responses to Antigenically Drifted Influenza A(H3N2) Viruses among Children and Adolescents following 2014-2015 Inactivated and Live Attenuated Influenza Vaccination
Abstract
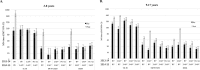
Human influenza A(H3N2) viruses that predominated during the moderately severe 2014-2015 influenza season differed antigenically from the vaccine component, resulting in reduced vaccine effectiveness (VE). To examine antibody responses to 2014-2015 inactivated influenza vaccine (IIV) and live-attenuated influenza vaccine (LAIV) among children and adolescents, we collected sera before and after vaccination from 150 children aged 3 to 17 years enrolled at health care facilities. Hemagglutination inhibition (HI) assays were used to assess the antibody responses to vaccine strains. We evaluated cross-reactive antibody responses against two representative A(H3N2) viruses that had antigenically drifted from the A(H3N2) vaccine component using microneutralization (MN) assays. Postvaccination antibody titers to drifted A(H3N2) viruses were higher following receipt of IIV (MN geometric mean titers [GMTs], 63 to 68; 38 to 45% achieved seroconversion) versus LAIV (MN GMT, 22; only 3 to 5% achieved seroconversion). In 9- to 17-year-olds, the highest MN titers were observed among IIV-vaccinated individuals who had received LAIV in the previous season. Among all IIV recipients aged 3 to 17 years, the strongest predictor of antibody responses to the drifted viruses was the prevaccination titers to the vaccine strain. The results of our study suggest that in an antigenically drifted influenza season, vaccination still induced cross-reactive antibody responses to drifted circulating A(H3N2) viruses, although higher antibody titers may be required for protection. Antibody responses to drifted A(H3N2) viruses following vaccination were influenced by multiple factors, including vaccine type and preexisting immunity from prior exposure.
Copyright © 2016 Levine et al.
Figures

References
-
- Grohskopf LA, Olsen SJ, Sokolow LZ, Bresee JS, Cox NJ, Broder KR, Karron RA, Walter EB, Centers for Disease Control and Prevention. 2014. Prevention and control of seasonal influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices (ACIP) – United States, 2014-15 influenza season. MMWR Morb Mortal Wkly Rep 63:691–697. - PMC - PubMed
-
- Grohskopf LA, Sokolow LZ, Olsen SJ, Bresee JS, Broder KR, Karron RA. 2015. Prevention and control of influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices, United States, 2015-16 influenza season. MMWR Morb Mortal Wkly Rep 64:818–825. doi:10.15585/mmwr.mm6430a3. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

