Solvation Thermodynamics of Oligoglycine with Respect to Chain Length and Flexibility
- PMID: 27558719
- PMCID: PMC5002085
- DOI: 10.1016/j.bpj.2016.07.013
Solvation Thermodynamics of Oligoglycine with Respect to Chain Length and Flexibility
Abstract
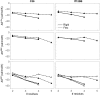
Oligoglycine is a backbone mimic for all proteins and is prevalent in the sequences of intrinsically disordered proteins. We have computed the absolute chemical potential of glycine oligomers at infinite dilution by simulation with the CHARMM36 and Amber ff12SB force fields. We performed a thermodynamic decomposition of the solvation free energy (ΔG(sol)) of Gly2-5 into enthalpic (ΔH(sol)) and entropic (ΔS(sol)) components as well as their van der Waals and electrostatic contributions. Gly2-5 was either constrained to a rigid/extended conformation or allowed to be completely flexible during simulations to assess the effects of flexibility on these thermodynamic quantities. For both rigid and flexible oligoglycine models, the decrease in ΔG(sol) with chain length is enthalpically driven with only weak entropic compensation. However, the apparent rates of decrease of ΔG(sol), ΔH(sol), ΔS(sol), and their elec and vdw components differ for the rigid and flexible models. Thus, we find solvation entropy does not drive aggregation for this system and may not explain the collapse of long oligoglycines. Additionally, both force fields yield very similar thermodynamic scaling relationships with respect to chain length despite both force fields generating different conformational ensembles of various oligoglycine chains.
Copyright © 2016 Biophysical Society. Published by Elsevier Inc. All rights reserved.
Figures




Similar articles
-
Thermodynamics of Conformational Transitions in a Disordered Protein Backbone Model.Biophys J. 2018 Jun 19;114(12):2799-2810. doi: 10.1016/j.bpj.2018.04.027. Biophys J. 2018. PMID: 29925017 Free PMC article.
-
Protein collapse driven against solvation free energy without H-bonds.Protein Sci. 2016 Jan;25(1):103-10. doi: 10.1002/pro.2749. Epub 2015 Aug 8. Protein Sci. 2016. PMID: 26174309 Free PMC article.
-
Solvation properties of N-substituted cis and trans amides are not identical: significant enthalpy and entropy changes are revealed by the use of variable temperature 1H NMR in aqueous and chloroform solutions and ab initio calculations.J Phys Chem A. 2005 Dec 29;109(51):11878-84. doi: 10.1021/jp0537557. J Phys Chem A. 2005. PMID: 16366639
-
Solvation free energies of alanine peptides: the effect of flexibility.J Phys Chem B. 2013 Dec 27;117(51):16428-35. doi: 10.1021/jp409693p. Epub 2013 Dec 13. J Phys Chem B. 2013. PMID: 24328358 Free PMC article.
-
Distinct role of hydration water in protein misfolding and aggregation revealed by fluctuating thermodynamics analysis.Acc Chem Res. 2015 Apr 21;48(4):956-65. doi: 10.1021/acs.accounts.5b00032. Epub 2015 Apr 6. Acc Chem Res. 2015. PMID: 25844814 Review.
Cited by
-
Revisiting the influence of pH on 1JCαH and chemical shifts of glycine and alanine short oligopeptides.R Soc Open Sci. 2023 Oct 4;10(10):230942. doi: 10.1098/rsos.230942. eCollection 2023 Oct. R Soc Open Sci. 2023. PMID: 37800158 Free PMC article.
-
Physical Chemistry of the Protein Backbone: Enabling the Mechanisms of Intrinsic Protein Disorder.J Phys Chem B. 2020 Jun 4;124(22):4379-4390. doi: 10.1021/acs.jpcb.0c02489. Epub 2020 May 14. J Phys Chem B. 2020. PMID: 32349480 Free PMC article. Review.
-
Thermodynamics of Conformational Transitions in a Disordered Protein Backbone Model.Biophys J. 2018 Jun 19;114(12):2799-2810. doi: 10.1016/j.bpj.2018.04.027. Biophys J. 2018. PMID: 29925017 Free PMC article.
-
Improvements to the ABSINTH Force Field for Proteins Based on Experimentally Derived Amino Acid Specific Backbone Conformational Statistics.J Chem Theory Comput. 2019 Feb 12;15(2):1367-1382. doi: 10.1021/acs.jctc.8b00573. Epub 2019 Jan 22. J Chem Theory Comput. 2019. PMID: 30633502 Free PMC article.
-
Intramolecular Interactions Overcome Hydration to Drive the Collapse Transition of Gly15.J Phys Chem B. 2017 Aug 31;121(34):8078-8084. doi: 10.1021/acs.jpcb.7b05469. Epub 2017 Aug 21. J Phys Chem B. 2017. PMID: 28774177 Free PMC article.
References
-
- Dunker A.K., Lawson J.D., Obradovic Z. Intrinsically disordered protein. J. Mol. Graph. Model. 2001;19:26–59. - PubMed
-
- Dunker A.K., Brown C.J., Obradovic Z. Intrinsic disorder and protein function. Biochemistry. 2002;41:6573–6582. - PubMed
-
- Uversky V.N., Santambrogio C., Grandori R. Length-dependent compaction of intrinsically disordered proteins. FEBS Lett. 2012;586:70–73. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

