Chronic electronic cigarette exposure in mice induces features of COPD in a nicotine-dependent manner
- PMID: 27558745
- PMCID: PMC5136722
- DOI: 10.1136/thoraxjnl-2015-208039
Chronic electronic cigarette exposure in mice induces features of COPD in a nicotine-dependent manner
Abstract
Background: The use of electronic (e)-cigarettes is increasing rapidly, but their lung health effects are not established. Clinical studies examining the potential long-term impact of e-cigarette use on lung health will take decades. To address this gap in knowledge, this study investigated the effects of exposure to aerosolised nicotine-free and nicotine-containing e-cigarette fluid on mouse lungs and normal human airway epithelial cells.
Methods: Mice were exposed to aerosolised phosphate-buffered saline, nicotine-free or nicotine-containing e-cigarette solution, 1-hour daily for 4 months. Normal human bronchial epithelial (NHBE) cells cultured at an air-liquid interface were exposed to e-cigarette vapours or nicotine solutions using a Vitrocell smoke exposure robot.
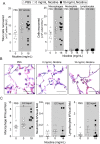
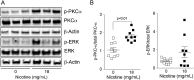
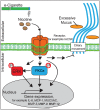
Results: Inhalation of nicotine-containing e-cigarettes increased airway hyper-reactivity, distal airspace enlargement, mucin production, cytokine and protease expression. Exposure to nicotine-free e-cigarettes did not affect these lung parameters. NHBE cells exposed to nicotine-containing e-cigarette vapour showed impaired ciliary beat frequency, airway surface liquid volume, cystic fibrosis transmembrane regulator and ATP-stimulated K+ ion conductance and decreased expression of FOXJ1 and KCNMA1. Exposure of NHBE cells to nicotine for 5 days increased interleukin (IL)-6 and IL-8 secretion.
Conclusions: Exposure to inhaled nicotine-containing e-cigarette fluids triggered effects normally associated with the development of COPD including cytokine expression, airway hyper-reactivity and lung tissue destruction. These effects were nicotine-dependent both in the mouse lung and in human airway cells, suggesting that inhaled nicotine contributes to airway and lung disease in addition to its addictive properties. Thus, these findings highlight the potential dangers of nicotine inhalation during e-cigarette use.
Keywords: COPD Pathology; COPD ÀÜ Mechanisms; Emphysema.
Published by the BMJ Publishing Group Limited. For permission to use (where not already granted under a licence) please go to http://www.bmj.com/company/products-services/rights-and-licensing/.
Conflict of interest statement
Competing interests: None declared.
Figures






Comment in
-
Electronic cigarettes: the lesser of two evils, but how much less?Thorax. 2016 Dec;71(12):1080-1081. doi: 10.1136/thoraxjnl-2016-209273. Thorax. 2016. PMID: 28441136 No abstract available.
References
-
- Murphy BS, Xu J, Kochanek KD. Deaths: preliminary data for 2010. In: Reports National Vital Statistics Report , ed. Hyattsville, MD: National Center for Health Statistics, 2012:61(4).
-
- Centers for Disease Control and Prevention (CDC). Cigarettes smoking among adults-United States, 2011. MMWR Morb Mortal Wkly Rep 2012;61:889–94. - PubMed
-
- US. Treating tobacco use and dependence: clinical practice guideline. In: Services DoHaH, ed. Department of Health and Human Services 2008 Report. 2008;1–32.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous
