Mincle Signaling Promotes Con A Hepatitis
- PMID: 27559045
- PMCID: PMC5026929
- DOI: 10.4049/jimmunol.1600598
Mincle Signaling Promotes Con A Hepatitis
Abstract
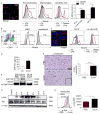
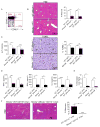
Con A hepatitis is regarded as a T cell-mediated model of acute liver injury. Mincle is a C-type lectin receptor that is critical in the immune response to mycobacteria and fungi but does not have a well-defined role in preclinical models of non-pathogen-mediated inflammation. Because Mincle can ligate the cell death ligand SAP130, we postulated that Mincle signaling drives intrahepatic inflammation and liver injury in Con A hepatitis. Acute liver injury was assessed in the murine Con A hepatitis model using C57BL/6, Mincle(-/-), and Dectin-1(-/-) mice. The role of C/EBPβ and hypoxia-inducible factor-1α (HIF-1α) signaling was assessed using selective inhibitors. We found that Mincle was highly expressed in hepatic innate inflammatory cells and endothelial cells in both mice and humans. Furthermore, sterile Mincle ligands and Mincle signaling intermediates were increased in the murine liver in Con A hepatitis. Most significantly, Mincle deletion or blockade protected against Con A hepatitis, whereas Mincle ligation exacerbated disease. Bone marrow chimeric and adoptive transfer experiments suggested that Mincle signaling in infiltrating myeloid cells dictates disease phenotype. Conversely, signaling via other C-type lectin receptors did not alter disease course. Mechanistically, we found that Mincle blockade decreased the NF-κβ-related signaling intermediates C/EBPβ and HIF-1α, both of which are necessary in macrophage-mediated inflammatory responses. Accordingly, Mincle deletion lowered production of nitrites in Con A hepatitis and inhibition of both C/EBPβ and HIF-1α reduced the severity of liver disease. Our work implicates a novel innate immune driver of Con A hepatitis and, more broadly, suggests a potential role for Mincle in diseases governed by sterile inflammation.
Copyright © 2016 by The American Association of Immunologists, Inc.
Conflict of interest statement
The authors have no conflicts of interest to declare.
Figures





References
-
- Chun LJ, Tong MJ, Busuttil RW, Hiatt JR. Acetaminophen hepatotoxicity and acute liver failure. J Clin Gastroenterol. 2009;43:342–9. - PubMed
-
- Singanayagam A, Bernal W. Update on acute liver failure. Curr Opin Crit Care. 2015;21:134–41. - PubMed
-
- Khashab M, Tector AJ, Kwo PY. Epidemiology of acute liver failure. Curr Gastroenterol Rep. 2007;9:66–73. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

