Electron anions and the glass transition temperature
- PMID: 27559083
- PMCID: PMC5018745
- DOI: 10.1073/pnas.1606891113
Electron anions and the glass transition temperature
Abstract
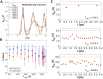
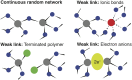
Properties of glasses are typically controlled by judicious selection of the glass-forming and glass-modifying constituents. Through an experimental and computational study of the crystalline, molten, and amorphous [Ca12Al14O32](2+) ⋅ (e(-))2, we demonstrate that electron anions in this system behave as glass modifiers that strongly affect solidification dynamics, the glass transition temperature, and spectroscopic properties of the resultant amorphous material. The concentration of such electron anions is a consequential control parameter: It invokes materials evolution pathways and properties not available in conventional glasses, which opens a unique avenue in rational materials design.
Keywords: amorphous materials; density functional theory; electrides; glass transition; molecular dynamics.
Conflict of interest statement
The authors declare no conflict of interest.
Figures












References
-
- Edwards PP, Sienko MJ. The transition to the metallic state. Acc Chem Res. 1982;15(3):87–93.
-
- Mott NF. Electrons in glass. Contemp Phys. 1977;18(3):225–245.
-
- Saitoh A, et al. Elucidation of codoping effects on the solubility enhancement of Er3+ in SiO2 glass: Striking difference between Al and P codoping. J Phys Chem B. 2006;110(15):7617–7620. - PubMed
-
- Berthier L, Ediger MD. Facets of glass physics. Phys Today. 2016;69(1):40–46.
-
- Dye JL. Chemistry. Electrons as anions. Science. 2003;301(5633):607–608. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

