P2X4 Receptor Reporter Mice: Sparse Brain Expression and Feeding-Related Presynaptic Facilitation in the Arcuate Nucleus
- PMID: 27559172
- PMCID: PMC4995303
- DOI: 10.1523/JNEUROSCI.1496-16.2016
P2X4 Receptor Reporter Mice: Sparse Brain Expression and Feeding-Related Presynaptic Facilitation in the Arcuate Nucleus
Abstract
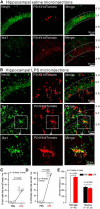
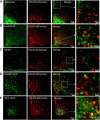
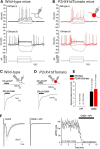
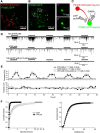
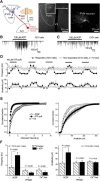
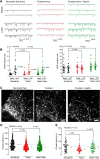
P2X4 receptors are ATP-gated cation channels that are widely expressed in the nervous system. To identify P2X4 receptor-expressing cells, we generated BAC transgenic mice expressing tdTomato under the control of the P2X4 receptor gene (P2rx4). We found sparse populations of tdTomato-positive neurons in most brain areas with patterns that matched P2X4 mRNA distribution. tdTomato expression within microglia was low but was increased by an experimental manipulation that triggered microglial activation. We found surprisingly high tdTomato expression in the hypothalamic arcuate nucleus (Arc) (i.e., within parts of the neural circuitry controlling feeding). Immunohistochemistry and genetic crosses of P2rx4 tdTomato mice with cell-specific GFP reporter lines showed that the tdTomato-expressing cells were mainly AgRP-NPY neurons and tanycytes. There was no electrophysiological evidence for functional expression of P2X4 receptors on AgRP-NPY neuron somata, but instead, we found clear evidence for functional presynaptic P2X4 receptor-mediated responses in terminals of AgRP-NPY neurons onto two of their postsynaptic targets (Arc POMC and paraventricular nucleus neurons), where ATP dramatically facilitated GABA release. The presynaptic responses onto POMC neurons, and the expression of tdTomato in AgRP-NPY neurons and tanycytes, were significantly decreased by food deprivation in male mice in a manner that was partially reversed by the satiety-related peptide leptin. Overall, we provide well-characterized tdTomato reporter mice to study P2X4-expressing cells in the brain, new insights on feeding-related regulation of presynaptic P2X4 receptor responses, and the rationale to explore extracellular ATP signaling in the control of feeding behaviors.
Significance statement: Cells expressing ATP-gated P2X4 receptors have proven problematic to identify and study in brain slice preparations because P2X4 expression is sparse. To address this limitation, we generated and characterized BAC transgenic P2rx4 tdTomato reporter mice. We report the distribution of tdTomato-expressing cells throughout the brain and particularly strong expression in the hypothalamic arcuate nucleus. Together, our studies provide a new, well-characterized tool with which to study P2X4 receptor-expressing cells. The electrophysiological studies enabled by this mouse suggest previously unanticipated roles for ATP and P2X4 receptors in the neural circuitry controlling feeding.
Keywords: ATP; P2X; arcuate; ion channel; mouse model; receptor.
Copyright © 2016 the authors 0270-6474/16/368903-19$15.00/0.
Figures




Similar articles
-
Ghrelin receptors mediate ghrelin-induced excitation of agouti-related protein/neuropeptide Y but not pro-opiomelanocortin neurons.J Neurochem. 2017 Aug;142(4):512-520. doi: 10.1111/jnc.14080. Epub 2017 Jun 21. J Neurochem. 2017. PMID: 28547758
-
Apolipoprotein A-IV Inhibits AgRP/NPY Neurons and Activates Pro-Opiomelanocortin Neurons in the Arcuate Nucleus.Neuroendocrinology. 2016;103(5):476-488. doi: 10.1159/000439436. Epub 2015 Aug 25. Neuroendocrinology. 2016. PMID: 26337236 Free PMC article.
-
Pharmacological and molecular characterization of ATP-sensitive K(+) conductances in CART and NPY/AgRP expressing neurons of the hypothalamic arcuate nucleus.Neuroscience. 2007 Feb 9;144(3):815-24. doi: 10.1016/j.neuroscience.2006.09.059. Epub 2006 Nov 28. Neuroscience. 2007. PMID: 17137725
-
AgRP/NPY and POMC neurons in the arcuate nucleus and their potential role in treatment of obesity.Eur J Pharmacol. 2022 Jan 15;915:174611. doi: 10.1016/j.ejphar.2021.174611. Epub 2021 Nov 17. Eur J Pharmacol. 2022. PMID: 34798121 Review.
-
The hypothalamic arcuate nucleus and the control of peripheral substrates.Best Pract Res Clin Endocrinol Metab. 2014 Oct;28(5):725-37. doi: 10.1016/j.beem.2014.03.003. Epub 2014 Apr 4. Best Pract Res Clin Endocrinol Metab. 2014. PMID: 25256767 Review.
Cited by
-
BAC transgenic mice to study the expression of P2X2 and P2Y1 receptors.Purinergic Signal. 2021 Sep;17(3):449-465. doi: 10.1007/s11302-021-09792-9. Epub 2021 May 28. Purinergic Signal. 2021. PMID: 34050505 Free PMC article.
-
Amino acid sensing in hypothalamic tanycytes via umami taste receptors.Mol Metab. 2017 Nov;6(11):1480-1492. doi: 10.1016/j.molmet.2017.08.015. Epub 2017 Sep 14. Mol Metab. 2017. PMID: 29107294 Free PMC article.
-
Preclinical evaluation of avermectins as novel therapeutic agents for alcohol use disorders.Psychopharmacology (Berl). 2018 Jun;235(6):1697-1709. doi: 10.1007/s00213-018-4869-9. Epub 2018 Mar 2. Psychopharmacology (Berl). 2018. PMID: 29500584 Free PMC article. Review.
-
TSPO: an emerging role in appetite for a therapeutically promising biomarker.Open Biol. 2021 Aug;11(8):210173. doi: 10.1098/rsob.210173. Epub 2021 Aug 4. Open Biol. 2021. PMID: 34343461 Free PMC article. Review.
-
Activation of basal forebrain purinergic P2 receptors promotes wakefulness in mice.Sci Rep. 2018 Jul 16;8(1):10730. doi: 10.1038/s41598-018-29103-4. Sci Rep. 2018. PMID: 30013200 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
