Comparative histopathology of the estrous or menstrual cycle in laboratory animals
- PMID: 27559240
- PMCID: PMC4963617
- DOI: 10.1293/tox.2016-0021
Comparative histopathology of the estrous or menstrual cycle in laboratory animals
Abstract
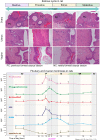
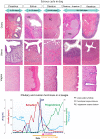
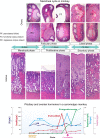
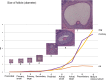
Accurate analysis of female reproductive toxicity requires a thorough understanding the differences in and specifics of estrous or menstrual cycles between laboratory animals. There are some species differences such as the time of sex maturation, the length of the estrous or menstrual cycle, the length of the luteal phase, the number of dominant follicles or corpora lutea, the size of follicles, processes of luteinization, and hormonal changes during the estrous or menstrual cycle. Rodents have a short estrous cycle, and their ovarian cycling features are the same in both ovaries, which contain a large number of follicles and corpora lutea. The dog estrous cycle is much longer than those of other laboratory animals, and it includes a long anestrus phase. The duration of the menstrual cycle of monkeys is roughly 30 days, and their ovarian cycling features are different between the left and right ovaries. In both rodents and dogs, the theca cells invade the early luteum, mixing with granulosa cells during luteinization. However in monkeys, the theca layer dose not mix with the granulosa cells as it invaginates only slightly into the early luteum. In addition, we found that high progesterone levels after ovulation are sustained for a much shorter duration in rodents than in dogs and monkeys due to the comparatively rapid passage of the rodent luteal phase. Based on these species differences, animal species for use in ovarian toxicology studies need to be selected appropriately.
Keywords: estrous cycle; female; gonadotropin; menstrual cycle; ovarian hormone; reproductive system.
Figures
Similar articles
-
Immunocytochemical localization of estradiol and progesterone receptors in the monkey ovary throughout the menstrual cycle.Endocrinology. 1988 Dec;123(6):2896-905. doi: 10.1210/endo-123-6-2896. Endocrinology. 1988. PMID: 3197647
-
Changes in ovarian function and gonadotropin secretion preceding the onset of nutritionally induced anestrus in Bos indicus heifers.Biol Reprod. 1996 Dec;55(6):1437-43. doi: 10.1095/biolreprod55.6.1437. Biol Reprod. 1996. PMID: 8949904
-
Corpus luteum function following single and double ovulation during estrous cycle in Sanjabi ewes.Anim Reprod Sci. 2009 Sep;114(4):362-9. doi: 10.1016/j.anireprosci.2008.10.011. Epub 2008 Nov 1. Anim Reprod Sci. 2009. PMID: 19042100
-
Luteal toxicity evaluation in rats.J Toxicol Pathol. 2022 Jan;35(1):7-17. doi: 10.1293/tox.2021-0058. Epub 2021 Nov 18. J Toxicol Pathol. 2022. PMID: 35221491 Free PMC article. Review.
-
The postpartum buffalo. II. Acyclicity and anestrus.Anim Reprod Sci. 2007 Feb;97(3-4):216-36. doi: 10.1016/j.anireprosci.2006.03.003. Epub 2006 Apr 18. Anim Reprod Sci. 2007. PMID: 16621354 Review.
Cited by
-
The antioxidative enzyme SOD2 is important for physiological persistence of corpora lutea in lynxes.Sci Rep. 2020 Feb 28;10(1):3681. doi: 10.1038/s41598-020-60634-x. Sci Rep. 2020. PMID: 32111948 Free PMC article.
-
Bisphenol A: an emerging threat to female fertility.Reprod Biol Endocrinol. 2020 Mar 14;18(1):22. doi: 10.1186/s12958-019-0558-8. Reprod Biol Endocrinol. 2020. PMID: 32171313 Free PMC article. Review.
-
Endocrine Disruption and Reproductive Pathology.Toxicol Pathol. 2019 Dec;47(8):1049-1071. doi: 10.1177/0192623319879903. Toxicol Pathol. 2019. PMID: 31833458 Free PMC article.
-
The influence of sex and reproductive cycle on cocaine-induced behavioral and neurobiological alterations: a review.Exp Brain Res. 2022 Dec;240(12):3107-3140. doi: 10.1007/s00221-022-06479-4. Epub 2022 Oct 20. Exp Brain Res. 2022. PMID: 36264315
-
Modulation of the lung inflammatory response to ozone by the estrous cycle.Physiol Rep. 2019 Mar;7(5):e14026. doi: 10.14814/phy2.14026. Physiol Rep. 2019. PMID: 30848106 Free PMC article.
References
-
- Greaves P. Female genital tract. In: Histopathology of Preclinical Toxicity Studies, 4th ed. Greaves P (ed). Academic Press, Amsterdam. 667–723. 2012.
-
- Andersson H, Rehm S, Stanislaus D, and Wood CE. Scientific and regulatory policy committee (SRPC) paper: assessment of circulating hormones in nonclinical toxicity studies III. female reproductive hormones. Toxicol Pathol. 41: 921–934. 2013. - PubMed
-
- Beckman DA, and Feuston M. Landmarks in the development of the female reproductive system. Birth Defects Res B Dev Reprod Toxicol. 68: 137–143. 2003; (Part B). - PubMed
-
- Liberati TA, Roe BJ, and Feuston MH. An oral (gavage) control embryo-fetal development study in the Wistar Hannover rat. Drug Chem Toxicol. 25: 109–130. 2002. - PubMed
-
- Yoshida M. Reproductive system. In: Ito’s Toxicologic Pathology. M Takahashi, and S Fukushima (eds). Maruzen, Japan. 321–334. 2013.
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
