Novel non-cyclooxygenase inhibitory derivatives of naproxen for colorectal cancer chemoprevention
- PMID: 27559271
- PMCID: PMC4993210
- DOI: 10.1007/s00044-014-0979-z
Novel non-cyclooxygenase inhibitory derivatives of naproxen for colorectal cancer chemoprevention
Abstract
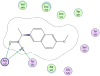
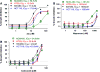
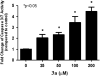
A structure-based medicinal chemistry strategy was applied to design new naproxen derivatives that show growth inhibitory activity against human colon tumor cells through a cyclooxygenase (COX)-independent mechanism. In vitro testing of the synthesized compounds against the human HT-29 colon tumor cell line revealed enhanced growth inhibitory activity compared to the parent naproxen with 3a showing IC50 of 11.4 μM (two orders of magnitude more potent than naproxen). Selectivity of 3a was investigated against a panel of three tumor and one normal colon cell lines and showed up to six times less toxicity against normal colonocytes. Compound 3a was shown to induce dose-dependent apoptosis of HT116 colon tumor cells as evidenced by measuring the activity of caspases-3 and 7. None of the synthesized compounds showed activity against COX-1 or COX-2 isozymes, confirming a COX-independent mechanism of action. Compound 3k was found to have no ulcerogenic effect in rats as indicated by electron microscope scanning of the stomach after oral administration. A pharmacophore model was developed for elucidating structure-activity relationships and subsequent chemical optimization for this series of compounds as colorectal cancer chemopreventive drugs.
Keywords: Apoptosis; Chemoprevention; Colorectal cancer; Cyclooxygenase; NSAIDs; Naproxen; Pharmcophore; Ulcerogenicity.
Figures




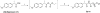
References
-
- Beck D, Roberts P, Rombeau J, Stamos M, Wexner S. Colorectal cancer: epidemiology, etiology, and molecular basis. In: Wexner SD, Stamos MJ, Rombeau J, Roberts PL, Beck DE, editors. The ASCRS manual of colon and rectal surgery. Springer; New York, New York: 2009. pp. 463–484. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
