Conjugate addition-enantioselective protonation reactions
- PMID: 27559372
- PMCID: PMC4979737
- DOI: 10.3762/bjoc.12.116
Conjugate addition-enantioselective protonation reactions
Abstract
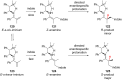
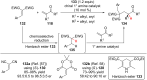
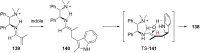
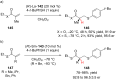
The addition of nucleophiles to electron-deficient alkenes represents one of the more general and commonly used strategies for the convergent assembly of more complex structures from simple precursors. In this review the addition of diverse protic and organometallic nucleophiles to electron-deficient alkenes followed by enantioselective protonation is summarized. Reactions are first categorized by the type of electron-deficient alkene and then are further classified according to whether catalysis is achieved with chiral Lewis acids, organocatalysts, or transition metals.
Keywords: asymmetric catalysis; conjugate addition; enantioselective protonation; enolate.
Figures

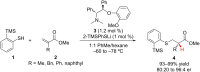
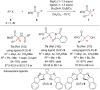
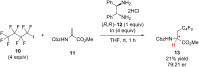
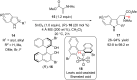


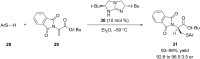








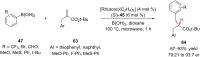
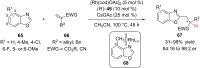
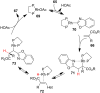

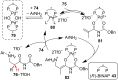

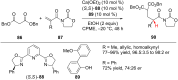

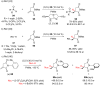

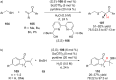





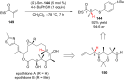


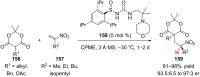


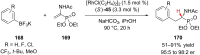



References
-
- Fehr C. Angew Chem, Int Ed Engl. 1996;35:2567–2587. doi: 10.1002/anie.199625661. - DOI
-
- Yanagisawa A, Ishihara K, Yamamoto H. Synlett. 1997:411–420. doi: 10.1055/s-1997-6131. - DOI
-
- Eames J, Weerasooriya N. Tetrahedron: Asymmetry. 2001;12:1–24. doi: 10.1016/S0957-4166(00)00496-1. - DOI
-
- Duhamel L, Duhamel P, Plaquevent J-C. Tetrahedron: Asymmetry. 2004;15:3653–3691. doi: 10.1016/j.tetasy.2004.09.035. - DOI
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
