Amidofluorene-appended lower rim 1,3-diconjugate of calix[4]arene: synthesis, characterization and highly selective sensor for Cu(2.)
- PMID: 27559419
- PMCID: PMC4979684
- DOI: 10.3762/bjoc.12.163
Amidofluorene-appended lower rim 1,3-diconjugate of calix[4]arene: synthesis, characterization and highly selective sensor for Cu(2.)
Abstract
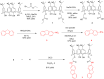
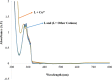
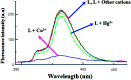
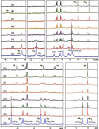
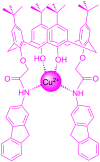
Functionalization of calix[4]arene with amidofluorene moieties at the lower rim led to formation of the 1,3-diconjugate of calix[4]arene L as a novel fluorescent chemosensor for Cu(2+). The receptor molecule L exhibited a pronounced selectivity towards Cu(2+) over other mono and divalent ions. The formation of the complex between L and Cu(2+) was evaluated by absorption, fluorescence and (1)H NMR spectroscopy. The sensor L showed a remarkable color change from colorless to purple and a fluorescence quenching only upon interaction with Cu(2+). The 1:1 stoichiometry of the obtained complex has been determined by Job's plot. The association constant determined by fluorescence titration was found to be 1.8 × 10(6) M(-1). The sensor showed a linear response toward Cu(2+) in the concentration range from 1 to 10 µM with a detection limit of 9.6 × 10(-8) M.
Keywords: calix[4]arene; chemosensor; copper ions; fluorene; fluorescence.
Figures





References
-
- de Silva A P, Fox D B, Huxley A J M, Moody T S. Coord Chem Rev. 2000;205:41–57. doi: 10.1016/S0010-8545(00)00238-1. - DOI
-
- Valeur B. Molecular Fluorescence. Weinheim, Germany: Wiley-VCH; 2001.
-
- Chang K-C, Luo L-Y, Diau E W-G, Chung W-S. Tetrahedron Lett. 2008;49:5013–5016. doi: 10.1016/j.tetlet.2008.06.060. - DOI
LinkOut - more resources
Full Text Sources
Other Literature Sources
